Abstract
Background
Renewable energy production is currently a major issue worldwide. Biogas is a promising renewable energy carrier as the technology of its production combines the elimination of organic waste with the formation of a versatile energy carrier, methane. In consequence of the complexity of the microbial communities and metabolic pathways involved the biotechnology of the microbiological process leading to biogas production is poorly understood. Metagenomic approaches are suitable means of addressing related questions. In the present work a novel high-throughput technique was tested for its benefits in resolving the functional and taxonomical complexity of such microbial consortia.
Results
It was demonstrated that the extremely parallel SOLiD™ short-read DNA sequencing platform is capable of providing sufficient useful information to decipher the systematic and functional contexts within a biogas-producing community. Although this technology has not been employed to address such problems previously, the data obtained compare well with those from similar high-throughput approaches such as 454-pyrosequencing GS FLX or Titanium. The predominant microbes contributing to the decomposition of organic matter include members of the Eubacteria, class Clostridia, order Clostridiales, family Clostridiaceae. Bacteria belonging in other systematic groups contribute to the diversity of the microbial consortium. Archaea comprise a remarkably small minority in this community, given their crucial role in biogas production. Among the Archaea, the predominant order is the Methanomicrobiales and the most abundant species is Methanoculleus marisnigri. The Methanomicrobiales are hydrogenotrophic methanogens. Besides corroborating earlier findings on the significance of the contribution of the Clostridia to organic substrate decomposition, the results demonstrate the importance of the metabolism of hydrogen within the biogas producing microbial community.
Conclusions
Both microbiological diversity and the regulatory role of the hydrogen metabolism appear to be the driving forces optimizing biogas-producing microbial communities. The findings may allow a rational design of these communities to promote greater efficacy in large-scale practical systems. The composition of an optimal biogas-producing consortium can be determined through the use of this approach, and this systematic methodology allows the design of the optimal microbial community structure for any biogas plant. In this way, metagenomic studies can contribute to significant progress in the efficacy and economic improvement of biogas production.
Similar content being viewed by others
Background
The utilization of fossil fuels on a global scale is limited by the availability of these resources and by the environmental effects of their excessive exploitation. The production of renewable energy carriers is therefore currently receiving increasing attention worldwide. Biogas is a promising candidate as the technology of its production may combine the treatment of various organic wastes with the generation of an energy carrier for the most versatile applications [1–4]. Biogas can be converted to heat and/or electricity, and its purified derivative, biomethane, is suitable for every function for which fossil natural gas is used today. The decomposition of organic materials by a microbial community is carried out under anaerobic conditions [5]. The great variety of diverse microbes that participate in the microbial food chain gradually degrade the complex molecules essentially to a mixture of CH4 and CO2[6–9]. The actions of the various microbes, involving members of the Eubacteria and Archaea, are coordinated by environmental and internal factors. The composition of this microbial consortium depends on various factors, such as substrate ingredients, temperature, pH, mixing, or the geometry of the anaerobic digester. A clear understanding of the organization and behavior of this multifarious community is crucial for optimization of their performance and attainment of the stable operation of the process. Classical microbiological methods are principally based on studies of isolated pure strains of microbes, and hence are of little help when the goal is elucidation of the relationships among members of a complex microbial consortium in order to improve the overall performance.
The developent of high-throughput sequencing technologies has opened up new avenues for such investigations. Methods with which to reveal the compositions of microbial communities, based on the generation of 16 S rRNA gene clone libraries and Sanger sequencing of the 16 S rDNA amplicons, have recently been devised [10–13]. Archaeal community members have been identified and semi-quantitatively enumerated through the use of the mcrA gene, which codes for one of the key enzymes in methanogenesis, the α-subunit of methyl-coenzyme M reductase occurring uniquely in methanogens [14]. Alterations in the organization of methanogenic communities under various conditions have been reported on the basis of this phylogenetic marker [15–19].
The automated Sanger sequencing approach is frequently referred to as “first generation sequencing”. The past few years have brought important technical breakthroughs and the “next-generation sequencing” techniques have been developed. A common feature of these methods, which employ various chemical reactions for the rapid determination of DNA sequences [20, 21], is the production of huge databases prepared from relatively short sequence fragments and the use of sophisticated bioinformatics to analyze the results [22]. This metagenomic approach allows the real-time study of live consortia in various environments through identification of the members of these communities [23–25] and/or determination of the relative abundances of particular physiological functions, reflected in the occurrence of specific enzymes [26–28]. Currently the most widespread next-generation sequencing method employs 454-pyrosequencing procedures for metagenomic purposes (Roche). This technique has been used for the characterization of biogas-producing communities [29–33], among numerous other applications. A fundamentally different methodology is offered by the SOLiD™ (s equencing by oligo li gation and d etection) technology (Applied Biosystems). As indicated by its name, SOLiD™ is based on a ligation reaction and each nucleotide is interrogated twice, which significantly reduces the potential errors arising from misreading and thereby improves the reliability of the data [34, 35]. Since its introduction onto the market in 2007, a number of systems have been investigated with the SOLiD™ method [36–39], but as far as we are aware biogas-producing microbial communities have not been analyzed by SOLiD™ so far. Besides its exceptional accuracy, the fundamental differences as compared with the 454-pyrosequencing approach are the extremely high throughput of the SOLiD system (200 Gb/run) and the short-read technology (50–75 nucleotides/read).
The aim of the present study was to determine the possibility of applying this short-read next-generation sequencing technology to characterize the composite microbial consortium developing in a biogas fermenter and to test whether the results validate those obtained by using the pyrosequencing approach. Samples were taken from an anaerobic fermenter fed primarily with plant biomass and pig manure slurry so that the conclusions could be compared with those drawn from other data sets relating to distinct anaerobic degradation processes with similar substrates.
Results and discussion
Distribution of metabolic functions in the microbial community
In order to gain an insight into the diverse biochemistry of the biogas-producing community, the short DNA sequences generated by parallel sequencing were used to create environmental gene tags (EGTs) and clusters of orthologous groups of proteins (COGs). The raw sequence reads of about 50 bp were assembled into contigs by using the CLC Bio Genomics Work Bench software [40]. The generated contigs were uploaded to the MG-RAST server, where the data were automatically normalized, processed and evaluated. Those that passed the quality control (see Materials and Methods) were aligned to sequences stored in a number of public databases [41]. This permits classification in the taxonomic and functional hierarchy. Figure 1. reflects the reliability of the results. 26,895 contigs passed the quality control. The contigs were translated into proteins, yielding 13,545 (52%) predicted protein sequences. 12,441 (91%) of the annotated features could be placed in the functional hierarchy. In this way, the DNA sequences from the SOLiD™ reads could be linked to metabolic functions. The results are depicted in Figure 2.
Source hit distribution. Legend: The graph displays the number of features in our examined dataset that were annotated by the different databases: GenBank- National Institutes of Health Genetic Sequence Database, IMG- Integrated Microbial Genomes at the Joint Genome Institute, KEGG- Kyoto Encyclopedia of Genes and Genomes, PATRIC- Pathosystems Resource Integration Center, RefSec- National Center for Biotechnology Information Reference Sequences Database, SEED- The SEED Project, SwissProt- Swiss-Prot Uniport Knowledgebase, TrEMBL- TrEMBL Uniport Knowledgebase, eggNOG- evolutionary genealogy of genes: Non-supervised Orthologous Groups, COG- eggNOG: Clusters of Orthologous Groups, KO- KEGG Orthology, NOG- eggNOG: Non-supervised Orthologous Groups, Subsystems- SEDD Subsystem Annotation, Greengenes- 16 S rRNA Gene Database, SILVA LSU- SILVA Large Subunit rRNA Database, RDP- Ribosomal Database Project, SILVA SSU- SILVA Small Subunit rRNA Database. The bars represent annotated reads, which are colored according to their e-value range
Functional hierarchical classification analysis. Legend: The graph shows the abundances of COGs in % using best hits of Subsystems protein database. The most abundant functions are related to biosynthesis, bioenergetics and housekeeping. The numbers on the top of the columns indicate filtered hits, for filtration rules see Material and Methods, data normalization and analysis section
Most of the COGs are linked to information storage and the basic metabolisms of the organic macromolecules (proteins, nucleic acids, lipids, and carbohydrates). Similarly, a large number of COGs related to the biosynthesis of basic cell components, such as cell wall material, vitamins, protective mechanisms and stress responses. These functions are required for the appropriate performance of the community, and therefore are expected to manifest themselves. The high numbers of protein and DNA metabolism COGs suggest that the cells are mostly active. Energy generation and storage are further representations of important functional groups of COGs. These findings are in line with previous studies which indicated that the housekeeping mechanisms and carbohydrate metabolism are predominant. Among the genes involved in the carbohydrate metabolism, those that degrade cellulose are particularly important for the efficient breakdown of the cellulosic biomass substrate. The 16 S rDNA hits and COGs demonstrated that the Firmicutes phylum is of outstanding importance in cellulose degradation by the biogas microbial community, corroborating earlier findings [29–31, 42].
Taxonomic profile of the biogas microbial community
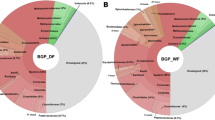
The assembled contigs were subjected to taxonomic analysis through use of the MG-RAST server [43]. The results were filtered for e-values, percentages of homology and lengths of homology. The ensuing identification and abundance list clearly showed that prokaryotes comprised the most abundant domain; the predominant systematic groups were the Bacteria and Archaea (Figure 3). Within the Bacteria domain, the Firmicutes phylum proved most abundant. The classes Clostridia and Bacilli belonging in this phylum accounted for the majority of the Bacteria in the biogas fermenter. In the Archaea domain, the Methanomicrobiales family provided a preponderance of the identified species. Members of the above-mentioned systematic groups have been identified previously in the anaerobic digestion of maize silage and silage supplemented with animal manure [29–31, 42]. It should be noted that a number of sequence reads did not exhibit homology to any of the known and sequenced microbial species, which implies the presence of numerous so far unidentified microbes in biogas fermenters.
Taxonomic distribution of the biogas community. Legend: Allocation of assembled contig sequences to microbial genome. Results were obtained by best M5nr database hits. Bacteria dominate the community, Archaea represent about 10% of the microbiome. Within Firmicutes the Clostridia stand out, among Archaea the hydrogenotrophic methanogens were found in highest number. The numbers in parentheses show the abundances, i.e. the number of sequence features with a hit. The figure was prepared by Krona interactive visualization program (offered by MG-RAST [44])
The bacteria domain
More than 1,000 representatives of the Bacteria domain were identified in the metagenomic database.
The first step in the anaerobic degradation of complex organic substrates involves the breakdown of large molecules by hydrolysis [45, 46]. Certain communities of bacteria are capable of the efficient hydrolysis of plant biomass rich in lignocellulose. Most of these bacteria belong in the classes of the Clostridia and Bacilli. As expected, the overwhelming majority of the identified abundant species in our biogas fermenter were members of the Clostridia (36%) and Bacilli (11%) classes, together with members of the Bacteroidia (3%), Mollicutes (3%), Gammaproteobacteria (3%) and Actinobacteria (3%) classes (Figure 3). Unassigned and unidentified sequences were ignored in this analysis. The most abundant identified species are listed in Table 1. and the presence or absence of cellulose degrading activity and hydrogenase enzymes is indicated.
Among the Clostridia, Clostridium thermocellum occurred most frequently. This species can hydrolyze cellulose efficiently by means of its extracellular cellulases, which are organized into cellulosomes [47, 48]. An outstanding member of this class is C. kluyveri, which is unique among the Clostridia, because it uses ethanol and acetate as sole energy sources and converts these substrates to butyrate and H2[49]. A prominent and well-characterized species is C. acetobutylicum, which exerts cellulolytic, saccharolytic and H2-producing activities. The fermentation pathways may yield organic acids such as acetate and butyrate (acetogenesis), or acetone, butanol and ethanol (solventogenesis) [50, 51]. C. perfingens generates lactate, acetate and butyrate from sugars, and through its [FeFe]-hydrogenase, it can also produce H2[52]. Similarly to C. thermocellum C. cellulolyticum is a well-known strain that degrades cellulose to acetate and evolves CO2 and H2[53]. C. saccharolyticum additionally possesses cellulolytic activity. The fermentation products include acetate, ethanol, H2 and CO2[54]. C. difficile is one of the rare pathogens [55] found in a biogas community. Thermoanaerobacterium thermosaccharolyticum is a H2-producing bacterium that has been reported to live in co-culture with C. thermocellum, the mixed culture producing more H2 than the pure cultures [56, 57]. Ruminococcus albus has been noted for its efficient cellulose-degrading activity by cellulosomes; the major fermentation product is ethanol [58]. Both Anaerotruncus colihominis and Faecalibacterium prausnitzki colonize the intestine and produce various volatile organic acids from glucose and acetate, respectively [59, 60].
Besides being capable of reductive dechlorination, Desulfitobacterium hafniense can produce sulfide from thiosulfate or sulfite, but cannot reduce sulfate. As carbon source it prefers to ferment pyruvate and lactate. This species is also known to contain Hup (h ydrogen-up take) type of [NiFe]-hydrogenases [61]. Heliobacterium modesticalum can grow in either photoheterotrophic or chemotrophic mode. Under chemotrophic conditions it ferments acetate to H2 and CO2. It also contains a number of hydrogenases, including [NiFe]- and [FeFe]-hydrogenases [62]. H2 and acetate are generated by Caldanaerobacter subterraneus from lactose, glucose or cellobiose as substrate [63]. Syntrophomonas wolferi ferments long-chain fatty acids and lives in co-culture with methanogenic Archaea [64]. Pelotomaculum thermopropionicum too forms a syntrophic relationship with methanogens, and its abundance in the anaerobic digester community is therefore reasonable. The syntrophic associations play important roles in efficient biogas formation [65]. The unique members of the Clostridia class, Alkaliphilus metalliredigens and Desulfotomaculum reducens, were detected in unexpectedly high amounts. These bacteria are known to use lactate and acetate as electron sources for the reduction of iron and cobalt in anaerobic respiration [66]. Although it may not be trivial to explain the occurrence of metal-reducing bacteria in an anaerobic biogas-producing community, it should be noted that these bacteria also possess highly active [FeFe]-hydrogenases [67]. Caldicellulosiruptor saccharolyticus is a cellulose-degrading and H2-producing bacterium. Addition of a pure culture of C. saccharolyticus to sewage sludge, plant biomass, animal manure or a mixture of these significantly increased the extent of biogas production [68]. Finegoldia magna has noteworthy substrate specificity, as it can utilize only fructose from among a range of sugars, and produces acetate [69]. F. magna also carries the genes for a putative hydrogenase [70]. The large number and proportion of members of the Clostridiales order are indicative of the important role of these bacteria in the proper functioning of the microbial community in an anaerobic digester fed with complex substrates. Their contribution to the breakdown of polysaccharide molecules may be explained by the high cellulolytic activity of numerous members of the Clostridiales order, and members of the Clostridiaceae family are capable of performing diverse fermentation pathways. They primarily ferment sugars to organic acids [71]. The Wood-Ljungdahl pathway, also known as the reductive acetyl-CoA pathway, plays an important role in this process, which is typical in acetogenic bacteria and in some Archaea [72]. In this process, CO2 is reduced to CO and then converted to acetyl-CoA, H2 serving as electron donor [73]. In the anaerobic digester, the aceticlastic Archaea split acetate to CH4 and CO2 in an energy gaining process [74]. Besides the acetogenic Clostridia discussed above, Moorella thermoacetica and Carboxidothermus hydrogenoformans also obtain energy via the Wood-Ljungdahl pathway. It should additionally be noted that a large number of Clostridia actively produce H2, an important substrate for the hydrogenotrophic methanogens. It is noteworthy that Cr. hydrogenoformans is able to use CO as carbon source as electron donor and water as an electron acceptor, to produce acetate and H2[75, 76]. Both Cr. hydrogenoformans and M. thermoacetica are capable of H2 production [77]. The predominance of the Clostridia in the anaerobic digester community triggers the activity of the hydrogenotrophic methanogens, which must keep the H2 partial pressure in the system low in order to ensure system stability [78]. The delicate balance between the Clostridia and hydrogenotrophic methanogens must be a determining factor within the biogas-producing microbial consortium (Figure 3).
The second largest group of bacteria in the anaerobic degradation community is the class of Bacilli in the Bacteria domain. The most abundant species from this class in our fermenters was Enterococcus faecalis. This strain, an anaerobic Gram-positive bacterium found in the digestive system, is able to hydrolyze plant polysaccharides and possesses hydrogenase activity in its formate dehydrogenase complex [79]. E. faecium is also common in the gastrointestinal system. These microbes convert carbohydrates such as fructose, maltose, lactose and galactose to acetate and ethanol [80, 81]. Bacillus cereus and B. thuringiensis can carry out both aerobic and anaerobic metabolism. Under anaerobic conditions, B. cereus ferments glucose to a mixture of acetate, lactate and ethanol, while B. thuringiensis produces mostly lactate [82, 83]. Streptococcus pneumonia is a pathogen that converts glucose to lactate [84]. Its relative S. suis can ferment glucose, lactose, maltose and trehalose to a mixture of volatile fatty acids [85], while S. agalactiae also generates ethanol beside the volatile acids [86, 87]. Additional pathogenic Bacilli detected in the anaerobic digester community, though in low abundance, include Staphylococcus epidermis and Listeria monocytogenes[88].
Over and above the members of the Clostridia and Bacilli classes discussed above, the study revealed additional members of the microbial systematic groups in the biogas-producing community, though their contribution to the microbiological food chain is probably limited relative to that of the Clostridia and Bacilli. Bacteroidia species were identified in meaningful quantities. Members of the Bacteriodia are common in nature at sites where degradable organic material is to be found, such as plants and other forms of biomass. Bacteroides capillosus is an intestinal bacterium that ferments lactate and produces H2, and also displays cellulolytic activity [89]. As an outstanding example of human-bacterium symbiosis, Bacteroides thetaiotamicron is a constituent of the intestinal flora, which specializes in hydrolyzing polysaccharides of plant origin, i.e. cellulose and starch, as carbon sources [90, 91]. Parabacteroides distasionis is a Gram-negative, non-spore-forming bacterium that produces volatile organic acids [92].
The members of the Mollicutes are facultative anaerobes. Under anaerobic conditions, they produce organic acids, which may be utilized by the acidoclastic methanogens [93]. Acoleplasmatales is the most abundant among the relatively few Mollicutes class members. Acoleplasma laidlawii ferments glucose to produce lactic acid, saturated fatty acids and acetate [94]. All these fermentation products are subsequently converted to biogas by the acetoclastic Archaea in the methanogenic consortium. Although Gammaproteobacteria are frequently found in diverse habitats, they do not appear to dominate in the biogas-producing community. Escherichia coli, one of the most widespread and certainly the most thoroughly studied bacterium, was present in the anaerobic community. E. coli, a facultative anaerobe, has a highly versatile metabolism. Under anaerobic conditions, it produces lactate, succinate, ethanol, acetate, H2 and CO2 in a mixed acid fermentation [95]. Various [NiFe]-hydrogenases are involved in the metabolism of H2 , and a syntrophic relationship often develops with H2 consumers in order to keep the H2 partial pressure low in the entire system [96]. Members of the Actinobacteria class are commonly found in soils and natural waters. Some of them effectively break down complex organic material such as cellulose, and thereby play an important role in the carbon cycle [97]. Furthermore, members of this group are known to produce lignin-degrading enzymes [98]. Two species of Actinobacteria were identified in our biogas fermenter samples: Slackia heliotrinireducens and Bifidobacterium longum. Sl. heliotrinireducens is a Gram-positive anaerobic bacterium which can reduce nitrate to ammonia if there are electron donors (H2 or formate) in the system. This organism has also been reported to produce acetic acid and lactic acid, and contains a hydrogenase [99, 100]. Bf. longum is a Gram-positive bacterium found as a symbiont in the human normal intestinal flora [101]. It metabolizes oligosaccharides and releases lactic acid, which helps control the normal microflora.
In addition to the known phylogenetic categories, 7% of the sequences belong to the Bacteria domain, but lacks detailed classification. In this group candidatus Cloacamonas acidaminovorans was found in remarkably high abundance. This species was also identified in several anaerobic digester microflora [31, 102]. c. Cm. acidaminovorans gains energy from sugars in the Embden-Meyerhof pathway and from the fermentation of amino acids. It is a fermentative H2 producer, containing a [FeFe]-hydrogenase, which is an indication of syntrophic metabolism [103].
The archaea domain
The volatile organic acids, CO2 and H2 generated by the acetogens are the substrates of methanogenesis carried out by special Archaea [104, 105]. Aceticlastic and hydrogenotrophic methanogens are distinguished in biogas fermentors [106]. The hydrogenotrophic Archaea are capable of reducing CO2 to CH4, H2 being used as an electron donor. The CO2-reducing pathway starts with the formation of N-carboxymethanofuran from CO2 and the C1-carrier methanofuran, which is subsequently reduced to formyl-methanofuran. The reductant is provided from reduced F420 (8-hydroxy-5-deazaflavin) and hydrogenases. The central electron carrier in hydrogenotrophic methanogenesis is coenzyme F420[107]. As the first step in the inverse Wood-Ljungdahl pathway, acetate is activated to acetyl-CoA with the participation of phosphotransacetylase and acetate kinase in acetotrophs [108]. Carbon monoxide dehydrogenase (CODH) then breaks down acetyl-CoA to CO, a methyl group and CoA [109]. CO is oxidized to CO2, which generates the electrons for reduction of the methyl radical to CH4[110].
Around 10% of the identified microbes in the biogas-producing community belonged in the Archaea (Figures 3 and 4). This correlated well with findings in previous studies [30, 42]. In the domain of the Archaea the Methanomicrobiales order predominates in the community. Within this order, the most abundant species is Methanoculleus marisnigri[111]. Interestingly, the same Archeon has been found in several methanogenic consortia [112, 113]. M. marisnigri JR1 is the only member of the Methanoculleus genus, which has been sequenced so far [114], and it cannot be excluded that several members of the same genus produce the high abundance of Methanoculleus-related reads [42]. Besides Methanoculleus, other representatives of Methanomicrobiales contribute to the plethora of hydrogenotrophic methanogens, e.g. Methanospirillum hungatei[115], Methanosphaerula palustris[116], Methanoregula boonei[117], Methanocorpusculum labreanum[118] and Methanoplanus petrolearius[119]. From the class of Methanococci, Methanococcus maripalidus is also a hydrogenotrophic methanogen [120] (Figure 5). Among the aceticlastic methanogens, Methanosarcina acetivorans[121] was present in a relative majority. An unidentified archaeon detected among rice rhizophere methanogens was also found in the anaerobic biogas community. This species was described as having a unique aerotolerant H2/CO2 dependent lifestyle and enzymes for carbohydrate metabolism and assimilatory sulfate reduction [122].
Energy and hydrogen metabolism related enzyme functions in the biogas producing community. Legend: The results were extracted from the Subsystem database. The numbers on the top of the columns indicate filtered hits, for filtration rules see Material and Methods, data normalization and analysis section
The predominance of the hydrogenotrophic methanogens strongly suggests that methane is generated mainly by the hydrogenotrophic pathway and aceticlastic methanogenesis plays a secondary role in the anaerobic digestion process (Figures 3 and 4.). H2 is produced for the hydrogenotrophic methanogens by the acetogens, e.g. Clostridia as shown above, or by syntrophic acetate oxidation [103, 123, 124]. At any rate the close proximity of the participating microbes and the very delicately balanced H2 metabolism are a must in these communities in order to keep the H2 concentration low and favor CH4 formation [68, 106]. Acetate stimulates the growth of Methanospirillum hungatei[115], Methanosphaerula. palustris[117], Methanoregula boonei[118], Methanocorpusculum labreanum[118], Methanococcus maripalidus[118] and Methanoplanus petrolearius[119]. In contrast, Methanoculleus marisnigri can only use CO2 as carbon source [110]. Accordingly, adequate acetate supply is required for the growth of hydrogenotrophic and aceticlastic methanogenesis and syntrophic acetate oxidizers [103, 118, 119, 121].
All of the identified Methanomicrobiales possess H2-activating membrane-associated hydrogenases [42, 117, 119, 125], and the relative wealth of hydrogenase-specific DNA reads corroborates the importance of these enzymes in the anaerobic degradation of organic material (Table 1 and Figure 5). Although the contributions of Eubacteria and Archaea cannot be distinguished in Figure 5, the widespread presence of H2-activating enzymes underlines their importance in the physiology of the biogas-producing community. A highly efficient interspecies H2 transfer [126] must take place between the H2-forming and consuming partners.
Besides the hydrogenases other genes encoding important redox proteins and likely to be connected to H2 metabolism were detected in the biogas fermenter, e.g. coenzyme M heterodisulfide heptanyl threonine phosphate (CoM-S-S-HTP) oxidoreductase, formate dehydrogenase and coenzyme F420 hydrogenase. CoM-S-S-HTP oxidoreductase catalyzes the conversion of CoM-S-S-HTP to HS-HTP (7-mercaptoheptanyl-L-threonine phosphate), which is a unique methanogenic cofactor in all methanogens [127]. Formate dehydrogenase extracts the hydrogen from formate and releases CO2[128]. Reduced F420 is oxidized by a membrane bound electron transport system. When F420 is oxidized, an equimolar amount of CoM-S-S-HTP is reduced. CoM-S-S-HTP oxidoreductase is common in all methanogens but formate dehydrogenase and coenzyme F420 are only typical to hydrogenotrophic methanogens [108].
Comparison of the 454-pyrosequencing and SOLiD™ metagenomic results
Previous studies designed to improve the understanding of microbial communities in biogas-producing anaerobic digestors, based on next-generation sequencing methods, relied exclusively on the pyrosequencing technique [29–31, 42]. The substrates fed into the fermentors included animal manure and green plant biomass (maize or green rye silage), commonly employed in German biogas facilities. Our laboratory fermenters were fed with a substrate mix with a similar composition, but our operational parameters, sample handling, DNA extraction protocols and sequence data collection and analysis methods were different.
The SOLiD™ sequencing method produces short individual reads (50 nucleotides) in a significantly higher number than does pyrosequencing. We have generated and analyzed 23,897,590 individual reads representing 1,194,879,500 bases. In previous studies, two versions of 454-pyrosequencing were employed and compared: GS FLX and Titanium [12]. The latter provides somewhat longer reads and increased throughput relative to GS FLX (454 GS FLX resulted in 616,072 sequence reads with an average read length of 230 bases, while Titanium resulted in 1,347,644 reads with an average read length of 368 bases). As a general rule of thumb, the longer the read sequence and the higher the number of independent reads, the more reliable the data.
In a comparison of the Bacteria domain, a remarkably good match was found between the data sets obtained by the various next-generation sequencing methods. In all cases, the class Clostridia comprised the most widespread group of microbes in the biogas fermenters. The Clostridia are noted for their highly effective cellulose degradation potential [129], and are therefore essential in the breakdown of lignocellulosic substrates in the biogas process. It should also be noted that the majority of Clostridia possess highly active hydrogenases. This is in line with the observation that hydrogenases have been found in large quantity among the redox enzymes in the biogas producing community (Figure 5.). Thus, the Clostridia may contribute to the widening of at least two bottlenecks in the biogas process, through the hydrolysis of large polymeric substrates and the in situ production of H2, an important reductant for the hydrogenotrophic methanogens [70, 130]. The positions of the most abundant strains in the methanogenic microbial food chain are summarized in Figure 6.
At the level of resolution of the abundances of individual strains, the most frequently occurring species likewise displayed a good correlation. Strains noted for their highly efficient polysaccharide degradation capabilities, such as Clostridium thermocellum, C. cellulolyticum and Caldicellulosiruptor saccharolyticus, are found to be the most abundant, regardless of the sequencing method used for their identification.
Similarly to the Bacteria, the members of the Archaea domain demonstrate a markedly comparable community structure, which is clearly reflected in any next-generation sequencing dataset. The analysis of the data at the species level revealed a strong correlation between the findings of the 454-pyrosequencing and SOLiD™ next-generation sequencing technology platforms. The Methanomicrobiales were indicated to constitute the majority of the Archaea in this environment by the sequencing with the 454 GS FLX [29–31], 454 Titanium [42] and SOLiD™ platforms alike. Within this taxon, the predominant genus is Methanoculleus, and the most abundant species according to our SOLiD™ results is M. marisnigri. Exactly the same picture was revealed by the 454-pyrosequencing approach [29–31, 42]. It is worth noting that the Methanomicrobiales are hydrogenotrophic methanogens, which are capable of reducing CO2 with H2 to produce additional CH4 in the biogas-producing consortium. The DNA-based community structure analysis of anaerobic degradation samples has already demonstrated the enormous importance of hydrogenotrophic methanogens.
Conclusions
The metagenomic analysis of biogas-producing microbial communities is a novel approach by which to study the complex interaction among microbes in an environment that is important for both basic research and the practical aspects of improvement of renewable energy production from biomass. In the present study, the Applied Biosystems’ SOLiD™ sequencing platform was used to collect relevant data. This next-generation DNA sequencing approach has not been used previously to characterize the microbial consortium of a biogas fermentor. Similar data sets determined with the Roche 454-pyrosequencer have been analyzed and reported [29–31, 42]. SOLiD™ differs from the 454 technique in several important technical aspects. SOLiD™ sequencing is based on ligation reactions, operates with a short read length and a much higher throughput than that of the 454 technique, and each nucleotide is read twice by the system, which makes the data highly accurate. Metagenomics is a special application and poses a real challenge since the complexity of the samples requires both high throughput and long reads. It is therefore important to compare the results obtained on a similar microbial community by using different analytical approaches; this can validate the various methodologies. It should be emphasized that a contribution is also made by microbes that are unknown or undetermined in the databases. These are not available for study by any of the current methods, but the rapid increase in available genome information justifies the exploitation of novel, high-throughput genomic methods in the field of community analysis.
One conclusion drawn from this study is that the sets of metagenomic information deduced from the databases via the various methods correlate well with each other. In this way, the databases generated through use of either of the investigated next-generation sequencing approaches have been validated and appear reliable and reproducible.
Although the anaerobic fermentation conditions (fermenter size, feedstock composition and origin, mixing, inoculum composition, etc.) were somewhat different, the SOLiD™ and 454-pyrosequencing data appear to lead to the same fundamental conclusions. Members of the Firmicutes and Bacteroides phyla play the most important role in the hydrolysis of the plant biomass and in the secondary fermentation. In particular, many Clostridium species were identified which possess cellulolytic and H2-producing activities, both properties probably being essential for the efficient degradation of the biomass. In the Archaea domain, Methanomicrobiales is the most abundant order that uses CO2 as a carbon source and H2 as an electron donor for methanogenesis. The predominance of the Methanomicrobiales and many hydrogenases suggests that the hydrogenotrophic pathway leading to CH4 formation may be more significant than recognized earlier [131–134]. Methanoculleus marisnigri proved to be the principal species among the archaeal habitants in the biogas fermenter. Interestingly, the same Archaeon has been identified as the most abundant in an anaerobic digester operated under different conditions [29–31, 42, 113, 114]. It is therefore concluded that an optimized balance between H2 producers and consumers is critical for the efficient operation of the biogas microbial community.
Methods
Fermentation conditions
The anaerobic digestion experiments were performed in 6-liter, continuously stirred tank reactors with a working volume of 5 liters. The fermenters were designed and constructed by Biospin Ltd, Hungary and installed at the Department of Biotechnology, University of Szeged [135]. The reactors were fed periodically with maize silage (68% oTS) added to pig manure slurry to sustain an average 15% oTS. Mixing of three fermenters operated in parallel was achieved with a single electronic engine through belt transmission in order to maintain identical mixing conditions. Heating was maintained by an electronically heated jacket which surrounded the cylindrical apparatus. Temperature was measured with a bimetallic-type sensor, and was maintained constant at 37 ± 1.0 °C. Electrodes for continuous monitoring of pH and redox potential were inserted into the fermentor in sealed sockets. The evolved gas left the fermentor through flexible neoprene tubing connected to the top plate, where ports for gas sampling through silicone rubber septa were also installed. Gas volume was measured with thermal mass flow controllers (DMFC, Brooks) attached to each gas exit port. The hydraulic retention time 60 days. The pH was maintained between 7.9-8.4. Acetate concentration was 0.1 g/mL, The volatile fatty acid content varied between 1.5 and 1.6 g HAceq/L, the buffering capacity was 9.21-9.28 g CaCO3/L. Data were collected, stored and analyzed with special software developed by Merat Ltd., Hungary. The key parameters (temperature, mixing speed and pH) were controlled continuously by the software. Biogas production was 610 LN/ kg oTS (organic total solids) with 52% methane content.
Purification of total DNA from biogas fermenter
A 2-ml liquid fermentation sample was utilized to prepare total community DNA by applying a CTAB based DNA extraction buffer [136–138]. Cell lysis was carried out at 55 °C overnight. Phenol:chloroform (1:1) was used to extract contamination, and the genomic DNA was precipitated with ethanol (90%). The DNA pellet was resuspended in 100 μl of TE buffer [139]. Its quantity was determined in a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Washington, USA). DNA purity was tested by agarose gelelectrophoresis. This method yielded a pure (A260/A280 = 1.8) and sufficient amount of total DNA (200–800 ng/μl).
Sequencing the DNA of the biogas fermenting microbial community
Sequencing was performing using an Applied Biosystems SOLiD™ 4 sequencing platform. Primary data analysis was carried out with software provided by the supplier (base-calling). The 50 nucleotide reads were analyzed, quality values for each nucleotide were determined, and the reads were assembled into contigs through use of the CLC Bio Genomics Workbench 4.6 program [40]. The preset parameters were as follows: minimum contig length = 200, similarity = 0.8, length fraction = 0.5, insertion cost = 3, deletion cost = 3, mismatch cost = 2, color space elignment = yes, color error cost = 3.
In the contig assembly process, 288 large contigs containing more than 1,000 bp were identified. The average length of the assembled contigs was 333 bp. The cumulative number of all contigs was 26,892, which amassed 8,978,367 bp. The contig size distribution is presented in Figure 7.
Data normalization and analysis
The assembled contigs were further analyzed by using the MG-RAST software package [140], which is a modified version of RAST (Rapid Annotations based on Subsystem Technology).
The MG-RAST server initially runs a quality control test. If the data appear reliable, the system automatically screens for sequences of potential protein encoding regions (PEGs) via a BLASTX [141] search against the SEED comprehensive non-redundant database compiled from various publicly available sequencing centers and other sources [142]. These databases include several rDNA datasets too, e.g. GREENGENES [143], RDP II [144], and European 16 S RNA [145], among other information sources. To identify the gene content of the biogas reactor, all contigs were functionally annotated by means of the c lusters of o rthologous g roups (COGs) of proteins made automatically by the MG-RAST server using the eggnog and COG databases. The generated matches to external databases were used to compute the derived data. The phylogenetic reconstruction of the contig sets was performed by using both the phylogenetic information contained in the SEED nr database and the similarities to the ribosomal RNA database. Functional classifications of the PEGs were computed by projecting against SEED FIGfams [146] and subsystems based on these similarity searches [142]. These functional assignments served as the raw input for an automatically generated initial metabolic reconstruction. The user interface provided a means of altering some of the parameters employed for the functional and metabolic reconstruction computation [140]. The acceptable percentage of identity was set to be >70%, the minimum read length was >35 nucleotides and the e-value cut-off was <10-6. The contigs formed from the sequence reads were compared with the M5nr database for phylogenetic analyses [147], which integrated the previously mentioned databases into a single, searchable database offered by MG-RAST.
Authors’ information
GM is Head of the Metagenomics Laboratory, Bay Zoltán Nonprofit Research Ltd., Szeged, Hungary. RW and EK are PhD students, ZB is a postdoc, RG is an Associate Professor, and KLK is a Full Professor and Department Chairman at the Department of Biotechnology, University of Szeged, Hungary. RG is the Director of the Environmental Research Institute at the University of Szeged. KLK is a Senior Adviser at the Institute of Biophysics, Biological Research Center, Hungarian academy of Sciences and also serves as President of the Hungarian Biogas Association.
Abbreviations
- SOLiD™:
-
Sequencing by Oligo Ligation and Detection new generation sequencing platform
- CTAB:
-
Cetyltrimethylammonium bromide
- EGT:
-
Environmental gene tags
- COG:
-
Orthologous group of proteins
- CoM-S-S-HTP:
-
Coenzyme M heterodisulfide heptanyl threonine phosphate) oxidoreductase
- HS-HTP:
-
7-mercaptoheptanyl-L-threonine phosphate
- PEGs:
-
Potential protein encoding genes
- TE buffer:
-
Tris-EDTA buffer (10 mM Tris, 1 mM EDTA pH: 8.0).
References
Angelidaki I, Ellegaard L: Codigestion of manure and organic wastes in centralized biogas plants: status and future trends. Appl Biochem Biotechnol 2003, 109: 95-105. 10.1385/ABAB:109:1-3:95
Daniels L: Biotechnological potential of methanogens. Biochem Soc Symp 1992, 58: 181-193.
Weiland P: Production and energetic use of biogas from energy crops and wastes in Germany. Appl Biochem Biotechnol 2003, 109: 263-274. 10.1385/ABAB:109:1-3:263
Santosh Y, Sreekrishnan TR, Kohli S, Rana V: Enhancement of biogas production from solid substrates using different techniques—a review. Bioresour Technol 2004, 95: 1-10. 10.1016/j.biortech.2004.02.010
Eder B, Schulz H: Biogas praxis: Grundlagen. Ökobuch Verlang GmbH, Planung, Anlagenbau, Beispiele, Wirtschaftlichkeit, Freiburg; 2005.
Bayer EA, Belaich JP, Shoham Y, Lamed R: The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol 2004, 58: 521-554. 10.1146/annurev.micro.57.030502.091022
Cirne DG, Lehtomaki A, Bjornsson L, Blackall LL: Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J Appl Microbiol 2007, 103: 516-527. 10.1111/j.1365-2672.2006.03270.x
Darke HL, Kusel K, Matthies C: Ecological consequences of the phylogenetic and physiological diversities of acetogens. Antonie Van Leeuwenhook 2002, 81: 203-213. 10.1023/A:1020514617738
Shin HS, Youn JH: Conversion of food waste into hydrogen by thermophilic acidogenesis. Biodegradation 2005, 16: 33-44. 10.1007/s10531-004-0377-9
Huang LN, Zhou H, Chen YQ, Luo S, Lan CY, Qu LH: Diversity and structure of the archaeal community in the leachate of a full-scale recirculating landfill as examined by direct 16S rRNA gene sequence retrieval. FEMS Microbiol Lett 2002, 214: 235-240. 10.1111/j.1574-6968.2002.tb11353.x
Klocke M, Mähnert P, Mundt K, Souidi K, Linke B: Microbial community analysis of a biogas-producing completely stirred tank reactor fed continuously with fodder beet silage as mono-substrate. Syst Appl Microbiol 2007, 30: 139-151. 10.1016/j.syapm.2006.03.007
McHugh S, Carton M, Mahony T, O’Flaherty V: Methanogenic population structure in a variety of anaerobic bioreactors. FEMS Microbiol Lett 2003, 219: 294-304.
Mladenovska Z, Dabrovski S, Ahring BK: Anaerobic digestion of manure and mixture of manure with lipids: biogas reactor performance and microbial community analysis. Water Sci Technol 2003, 48: 271-278.
Ferry JG: Enzymology of one-carbon metabolism in methanogenic pathways. FEM Microbiol. Rev 1999, 23: 13-38. 10.1111/j.1574-6976.1999.tb00390.x
Juottonen H, Galand PE, Yrjala K: Detection of methanogenic Archaea in peat: comparison of PCR primers targeting the mcrA gene. Res Microbiol 2006, 157: 914-921. 10.1016/j.resmic.2006.08.006
Luedeers T, Chin KJ, Concard R, Friedrich M: Molecular analysis of methyl – coenzyme M reductase alpha subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ Microbiol 2001, 3: 194-204. 10.1046/j.1462-2920.2001.00179.x
Luton PE, Wayne JM, Sharp RJ, Riley PW: The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 2002, 148: 3521-3530.
Nunoura T, Oida H, Miyazaki J, Miyashita A, Imachi H, Takai K: Quantification of mcrA by fluorescent PCR in methanogenic and methanotropic microbial communities. FEMS Microbiol Echol 2008, 64: 240-247. 10.1111/j.1574-6941.2008.00451.x
Zou C, Zhang J, Tang Y, Zhengkai X, Song R: Diversity of methanogenic archaea in biogas reactor fed with swine feces as the mono-substrate by mcrA analysis. Microbiol Res 2010, 1: 27-35.
MacLean D, Jonathan DG, Studhole JD: Application of „next- generation” sequencing technologies to microbial genetics. Nature Rev 2009, 7: 287-295.
Metzker ML: Sequencing technologies – the next generation. Nature Rev 2009, 11: 31-44.
Raes J, Foerstner KU, Bork P: Get the most out of your metagenome: computational analysis of environmental sequence data. Curr Opin Microbiol 2007, 10: 490-498. 10.1016/j.mib.2007.09.001
Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, Richardson PM, Solovyev VV, Rubin EM, Rokhsar DS, Banfield JF: Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 2004, 428: 37-43. 10.1038/nature02340
Venter JC, Adams MD, Myers EW, Li WP, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Codira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, et al.: The sequence of the human genome. Science 2001, 291: 1304-1351. 10.1126/science.1058040
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nelson K, White O, Peterson J, Hoffman J, Persons R, Baden-Tillson H, Pfannkoch C, Rogers Y-H, Smith HO: Environmental genome shotgun sequencing of the Sargasso Sea. Science 2004, 304: 67-74.
Angly FE, Felts B, Breitbart M, Salamon P, Edwards RA, Carlson C, Chan AM, Haynes M, Kelley S, Liu H, Mahaffy JM, Mueller JE, Nulton J, Olson R, Parsons R, Rayhawk S, Suttle CA, Fohwer F: The marine viromes of four oceanic regions. PloS Biol 2006, 4: e368. 10.1371/journal.pbio.0040368
Edwards RA, Rodrigez-Brito B, Wegley L, Haynes M, Breitbart M, Peterson DM, Saar MO, Alexander S, Rohwer F: Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genomics 2006, 7: 57. 10.1186/1471-2164-7-57
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE: Metagenomic analysis of the human distal gut microbiome. Science 2006, 312: 1355-1359. 10.1126/science.1124234
Schlüter A, Bekel T, Diaz NN, Dondrup M, Eichenlaub R, Gartemann KH, Krahn I, Krause L, Krömeke H, Kruse O, Mussgnug JH, Neuweger H, Niehaus K, Pühler A, Runte KJ, Szczepanpwski R, Tauch A, Tilker A, Viehöver P, Goessmann A: The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analyzed by the 454-pyrosequencing technology. J Biotech 2008, 136: 77-90. 10.1016/j.jbiotec.2008.05.008
Krause L, Diaz NN, Edwards RA, Gartemann K-H, Krömeke H, Neuwger H, Pühler A, Runte KJ, Schlüter A, Stoye J, Szczepanowski R, Tauch A, Goesmann A: Taxonomic composition and gene content of a methane-producing microbial community isolated from a biogas reactor. J Biotech 2008, 136: 91-101. 10.1016/j.jbiotec.2008.06.003
Kröber M, Bekel T, Diaz NN, Goesmann A, Sebastian J: Phylogenetic characterization of a biogas plant microbial community integrating clone library 16S-rDNA sequences and metagenome sequence data obtained by 454-pyrosequencing. J Biotech 2009, 142: 38-49. 10.1016/j.jbiotec.2009.02.010
Sundberg C, Abu Al-Soud W, Larsson M, Svennson B, Sörensson S, Karlsson A: 454-pyrosequencing analyses of bacterial and metagenomic Archaea DNA and RNA diversity in 20 full-scale biogas digesters. In Proceedings of the First International Conference on Biogas Microbiology: 14–16 September 2011; Leipzig. Edited by: Kleinsteuber S, Nikolausz M. UFZ Press, Leipzig; 2011:47.
Hanreich A, Schimpf U, Zakrzewski M, Schlüter A, Bendorf D, Klocke M: Monitoring of changes within a microbial, biogas producing community. In Proceedings of the First International Conference on Biogas Microbiology: 14–16 September 2011; Leipzig. Edited by: Kleinsteuber S, Nikolausz M. UFZ Press, Leipzig; 2011:49.
Mardis ER: The impact of next-generation sequencing technology on genetics. Trends in Gen 2008, 24: 133-141. 10.1016/j.tig.2007.12.007
Overview of SOLiD™ sequencing chemistry. [http://www.appliedbiosystems.com/absite/us/en/home/applications-technologies/solid-next-generation-sequencing/next-generation-systems/solid-sequencing-chemistry.html]
Liu Z, Klatt CG, Wood JM, Rusch DB, Ludwig M, Wittekindt N, Tomsho LP, Schuster SC, Ward DM, Bryant DA: Metatranscriptomic analyses of chlorophototrophs of a hot-spring microbial mat. IJSEM 2011, 5: 1279-1290.
Tyler HL, Roesch LFW, Gowda S, Dawson WO, Triplett EW: Confirmation of the sequence of ‘Candidatus liberibacter asiaticus’ and assessment of microbial diversity in Huanglongbing-infected Citrus phloem using a metagenomic approach. Mol Plant-Microbe Interactions 2009, 22: 1624-1634. 10.1094/MPMI-22-12-1624
Johansen SD, Karlsen BO, Furmanek T, Andreassen M, Jørgensen TE, Bizuayehu TT, Breines R, Emblem A, Kettunen P, Luukko K, Edwardsen RB, Nordeide JT, Coucheron DH, Moum T: RNA deep sequencing of the Atlantic cod transcriptome. Comp Biochem Physiol Part D: Genomics and Proteomics 2011, 6: 18-22. 10.1016/j.cbd.2010.04.005
McKernan KJ, Peckham HE, Costa G, McLaughlin S, Tsung E, Fu Y, Clouser YC, Dunkan C, Ichikawa J, Lee C, Zhang Z, Sherdian A, Fu H, Ranade S, Dimilanta E, Sokolsky T, Zhang L, Hendrickson C, Li B, Kotler L, Stuart J, Malek J, Manning J, Antipova A, Perez D, Moore M, Hayashibara K, Lynos M, Beaudoin R, Coleman B, et al.: Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two base encoding. Genome Res 2009, 19: 1527-1541. 10.1101/gr.091868.109
CLC Genomics Workbench. [http://www.clcbio.com/index.php?id = 1297]
Tools and data used in MG-RAST. [http://blog.metagenomics.anl.gov/tools-and-data-used-in-mg-rast/]
Jaenicke S, Ander C, Bekel T, Bisdorf R, Dröge M, Gartemann K-H, Jünemann S, Kaiser O, Krause L, Tille F, Zakrzewski F, Pühler A, Schlüter A, Goesmann A: Comparative and joint analysis of two metagenomic datasets from a biogas fermenter obtained by 454-pyrosequencing. PLoS One 2011, 6: e14519. 10.1371/journal.pone.0014519
About MG-RAST. [http://blog.metagenomics.anl.gov/about/]
Ondov BD, Bergman NH, Phillippy AM: Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 2011, 12: 385. 10.1186/1471-2105-12-385
Lynd L, Weimer P, van Zyl W, Pretorius I: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 2002, 66: 506-577. 10.1128/MMBR.66.3.506-577.2002
Drake H, Küsel K, Matthies C: Ecological consequences of the phylogenetic and physiological diversities of acetogens. Antonie Van Leeuwenhoek 2002, 81: 203-213. 10.1023/A:1020514617738
Zverlov VV, Kellermann J, Schwarz WH: Functional subgenomics of Clostridium thermocellum cellulosomal genes: identification of the major catalytic components in the extracellular complex and detection of three new enzymes. Proteomics 2005, 5: 3646-3653. 10.1002/pmic.200401199
Gold DN, Martin JJV: Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J Bacteriol 2007, 189: 6787-6795. 10.1128/JB.00882-07
Seedorf H, Fricke WF, Veith B, Brüggemann H, Liesegang H, Strittmatter A, Miethke M, Buckel W, Hinderberger J, Li F, Hagemeier C, Thauer RK, Gottschalk G: The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc Natl Acad Sci USA 2008, 105: 2128-2133. 10.1073/pnas.0711093105
Jones DT, Woods DR: Acetone-butanol fermentation revisited. Microbiol Mol Biol Rev 1986, 50: 484-524.
Sabathé F, Bélaїch A, Soucaille P: Characterization of the cellulolytic complex (cellulosome) of Clostridium acetobutylicum. FEMS Microbiol Lett 2002, 217: 15-22. 10.1111/j.1574-6968.2002.tb11450.x
Kaji M, Taniguchi Y, Matsushita O, Katayama S, Miyata S, Morita S, Okabe A: The hydA gene encoding the H2 evolving hydrogenase of Clostridium perfingens: molecular characterization and expression of the gene. FEMS Microbiol Lett 1999, 181: 329-336. 10.1111/j.1574-6968.1999.tb08863.x
Guedon E, Desvaux M, Petitdemange H: Improvement of cellulolytic properties of Clostridium cellulolyticum by metabolic engineering. Appl Environ Microbiol 2002, 68: 53-58. 10.1128/AEM.68.1.53-58.2002
Murray DW, Khan WA, van den Berg L: Clostridium saccharolyticurn sp. nov., a saccharolytic species from sewage sludge. IJSEM 1982, 32: 132-135.
Larson H, Price A, Honour P, Boriello S: Clostridium difficile and the etology of pseudomembranous colitis. The Lancet 1978, 311: 1063-1066. 10.1016/S0140-6736(78)90912-1
Shin H-S, Yaun J-H, Kim S-H: Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int. J. Hydrogen Energy 2004, 29: 1355-1363. 10.1016/j.ijhydene.2003.09.011
Liu Y, Yu P, Song X, Qu J: Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int J Hydrogen Energy 2008, 33: 2927-2933. 10.1016/j.ijhydene.2008.04.004
Mutolik S, Vinodkumar CS, Swamy S, Manjappa S: Depolymerization of bagasse by Ruminococcus albus in the production of eco-friendly fuel. Res Biotechnol 2011, 2: 1-6.
Lawson PA, Song Y, Chengxu L, Denise RM, Vaisanen ML, Collins MD, Finegold SM: Anaerotruncus colihominis gen. nov. sp. nov. from human feces. IJSEM 2004, 54: 413-417.
Duncan SH, Hold GL, Harmsen HJM, Stewart CS, Flimt HJ: Growth requirements and fermentation products of Fusobacterium prausnitzii and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov. comb. nov. IJSEM 2002, 52: 2141-2146.
Nokaga H, Keresztes G, Shinoda Y, Ikenaga Y, Abe M, Naito K, Inatomi K, Kensuke F, Inui M, Yukawa H: Complete genome sequence of the dehalorespiring bacteriumDesulfitobacterium hafnienseY51 and comparison withDehalococcoides ethenogenes195 J. Bacteriol 2006, 188: 2262-2274. 10.1128/JB.188.6.2262-2274.2006
Tang K-H, Yue H, Blankenship RE: Energy metabolism of Heliobacterium modesticaldum during phototrophic and chemotrophic growth. BMC Microbio 2010, 10: 150-164. 10.1186/1471-2180-10-150
Yokoyama H, Moriya M, Ohmori H, Waki M, Ogimo A, Tanaka Y: Community analysis of hydrogen producing extreme thermophilic anaerobic microflora enriched from cow manure with five substrates. Appl Microbiol Biotechnol 2007, 77: 213-222. 10.1007/s00253-007-1144-0
McInerney MJ, Briant MP, Hespell RB, Casterton JW: Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol 1981, 41: 1029-1039.
Schink B: Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 1997, 61: 262-280.
Ye Q, Roh Y, Caroll SL, Blair B, Zhou J, Zhang CL, Fields MW: Alkaline anaerobic respiration: isolation and characterization of a novel alkaliphilic and metal-reducing bacterium. Appl Environ Microbiol 2004, 70: 5595-5602. 10.1128/AEM.70.9.5595-5602.2004
Hydrogenases of Desulfotomaculum reducens.. [http://www.ncbi.nlm.nih.gov/gene?term = Desulfotomaculum%20reducens%20fe%20fe%20hydrogenase]
Bagi Z, Ács N, Bálint B, Horváth L, Dobó K, Perei RK, Rákhely G, Kovács KL: Biotechnological intensification of biogas production. Appl Microbiol Biotechnol 2007, 76: 473-482. 10.1007/s00253-007-1009-6
Goto T, Yamashita A, Hirakava H, Matsutani M, Todo K, Ohsima K, Toh H, Miyamato K, Kuhara S, Hattori M, Shimizu T, Akimoto S: Complete genome sequence of Finegoldia magna, an opportunitic pathogen. DNA Res 2008, 15: 39-47. 10.1093/dnares/dsm030
Finegoldia magna putative hydrogenase. [http://www.uniprot.org/uniprot/B0S2U0]
Demian AL, Newcomb M, David Wu JH: Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev 2005, 69: 124-154. 10.1128/MMBR.69.1.124-154.2005
Müller V: Energy conservation in acetogenic bacteria. App Environ Microbiol 2003, 69: 6345-6353. 10.1128/AEM.69.11.6345-6353.2003
Rother M, Oelgeschläger E: Carbon monoxide-dependent energy metabolism in anaerobic bacteria. Arch Microbiol 2008, 190: 257-269. 10.1007/s00203-008-0382-6
Ragsdale SW, Pierce E: Acetogenesis and the Wood- Ljungdahl pathway of CO2 fixation. Rev Biochim Biophys Acta 2008, 1784: 1873-1898. 10.1016/j.bbapap.2008.08.012
Wu M, Ren Q, Durkin AS, Daugherty SC, Brinkac LM, Dobson RJ, Madupu R, Sullivan SA, Kolonay JF, Nelson WC, Tallon LJ, Jones KM, Ulrich LE, Gonzalez JM, Zhullin IB, Robb FT, Eissen JA: Life in hot carbon monoxide: The complete genome sequence of Carboxydothermus hydrogenoformans. PloS Genet 2005, 1: 563-574.
Debarati P, Frank WA, Arick T, Bridges SM, Burgess SC, Yoginder SD, Lawrence ML: Genome sequence of the solvent-producing bacterium Clostridium carboxidivorans strain P7. J Bacteriol 2010, 192: 5554-5555. 10.1128/JB.00877-10
Pierche E, Xie G, Baradote RD, Saunders E, Hann SC, Detter JC, Richardson P, Brettin TS, Das A, Ljungdahl LG, Ragsdale SW: The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ Microbiol 2008, 10: 2550-2573. 10.1111/j.1462-2920.2008.01679.x
Boone DR, Whitman WB, Rouviere P: Diversity and taxonomy of methanogens. In Methanogenesis ecology, physiology, biochemistry and genetics. Edited by: Ferry JG. Chapman and Hall, New York; 1993:35-80.
Enterococcus faecalis hydrogenase 3. [http://biocyc.org/EFAE749511-HMP/NEWIMAGE?object = Hydrogenase-3]
Wei C, Kunio O, Shoichi S: Intergeneric protoplast fusion between Fusobacterium varium and Enterococcus faecium for enhancing dehydrodivanillin degradation. Appl Environ Microbiol 1987, 53: 542-548.
Guerra NP, Fajardo P, Fuciños C, Amado RI, Alonso E, Torrado A, Pastrana L: Modelling the biphasic growth and product formation by Enterococcus faecium CECT 410 in realkalized fed-batch fermentations in whey. J Biomed Biotechnol 2010. 290286. Epub.: 2010. 06. 10
Duport C, Zigha A, Ronsenfeld E, Schmitt P: Control of enterotoxin gene expression in Bacillus cereus F4430/73 involves the redox sensitive ResDE signal transduction system. J Bacteriol 2006, 188: 6640-6651. 10.1128/JB.00702-06
Ohara H, Yahata M: α-lactic acid production by Bacillus sp. in anaerobic and aerobic culture. J Ferm Bioeng 1996, 81: 272-274. 10.1016/0922-338X(96)82222-7
Hoskins J, Alborn JrWE, Arnold J, Blaszczak LC, Burgett S, DeHoff SB, Strem ST, Fritz L, Fu DJ, Fuller W, Geringer C, Gilmour R, Glass JS, Khoja H, Kraft AR, Lagace RE, LeBlanc DJ, Lee LN, Lefkowitz EJ, Lu J, Matshushima P, McAhren SM, McHenney M, McLeaster K, Mundy CW, Nicas TI, Norris FH, O’Gara M, Peery RB, Robertson GT, et al.: Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol 2001, 183: 5709-5717. 10.1128/JB.183.19.5709-5717.2001
Klipper-Balz R, Schleifer HK: Streptococcus suis sp. nov., nom. rev. IJSEM 1987, 37: 160-162.
Glaser P, Rusniak C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zauine M, Couvé E, Lalioui L, Poyort C, Trieu-Cout P, Kunst F: Genome sequence of Streptococcus agalactiae. Mol Microbiol 2002, 45: 1499-1513. 10.1046/j.1365-2958.2002.03126.x
Streptococcus agalactiae. [http://microbewiki.kenyon.edu/index.php/Streptococcus_agalactiae]
Ramick TL, Fleming HP, McFeeters RF: Anaerobic and aerobic metabolism of Listeria monocytogenes in different glucose media. Appl Environ Microbiol 1996, 62: 304-307.
Betian HG, Linehan BA, Bryant MP, Holdeman LV: Isolation of Bacteroides sp. from human feces. Appl Environ Microbiol 1977, 33: 1009-1010.
Bjursel MK, Martens EC, Gordon JI: Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J Biol Chem 2006, 281: 36269-36279. 10.1074/jbc.M606509200
Bacteroides thetaiotamicron. [http://microbewiki.kenyon.edu/index.php/Bacteroides_thetaiotaomicron]
Sakamato M, Benno Y: Reclassification of Bacteroides distasionis Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasionis gen. nov. comb. nov. Parabacteroides goldsteinii comb. nov. and Parabacteroides mardae comb. nov. IJSEM 2006, 56: 1599-1605.
Maniloff J: McElhaney RN, Finch LR, Baseman JB: Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington DC; 1992.
Lazarev VN, Levitskii SA, Basovskii YI, Chukin MM, Akopian TA, Vereshchagin VV, Kostrjukova ES, Kovaleva GY, Kazanov MD, Malko DB, Vitreschak AG, Sernova NV, Gelfand MS, Demina IA, Serebryakova MA, Galyamina MA, Vtyurin NN, Rogov SI, Alexeev DG, Ladygina VG, Govorun VN: Complete genome and proteome of Acoleplasma laidlawii. J Bacteriol 2011, 193: 4943-4953. 10.1128/JB.05059-11
Han K, Lim HC, Hong : Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng 1992, 15: 663-671.
Menon NK, Chatelus CY, Dervartanian M, Wendt JC, Shanmugam KT, Peck HD, Przybyla AE: Cloning and mutational analysis of the hub operon encoding Escherichia coli hydrogenase 2. J Bacteriol 1994, 176: 4416-4423.
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald FG, Chater FK, van Sinderen D: Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev 2007, 71: 495-548. 10.1128/MMBR.00005-07
Kirby B, Le Roes M, Meyers P: Kribella karoonensis sp. nov. and Kribella swartbargensis sp. nov., isolated from soil from the Western Cape, South Africa. IJSEM 2006, 56: 1097-1101.
Pukal R, Lapidus A, Nolan M, Copeland A, Glavina Del Rio T, Lucas S, Chen F, Tice H, Cheng J-F, Chertov O, Bruce D, Goodwin L, Kuske C, Brettin T, Detter JC, Han C, Pitluck S, Pati A, Mavrommatis K, Ivanova N, Ovchinnikova G, Chen A, Palaniappan K, Schneider S, Rohde M, Chain P, D’haeseleer P, Göker M, Bristow J, et al.: Complete genome sequence of Slackia heliotrinireducens type strain (RHS 1). SIGS 2009, 1:.
Slackia heliotrinireducens Fe-S-cluster-containing hydrogenase subunit. [http://www.uniprot.org/uniprot/C7N224]
Schell MA, Karmrabűntzon M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen M-C, Desiere F, Bork P, Delley M, Pridmore RD, Arigoni F: The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci USA 2002, 99:.
Pelletier E, Kreimeyer A, Bocs S, Rouy Z, Gyapay G, Chouari R, Rivière D, Ganesan A, Daegelen P, Sghir A, Cohen GN, Médigue C, Weissenbach J, La Paslier D: Candidatus Cloacamonas acidaminovorans”: genome sequence reconstruction provides a first glimpse of a new bacterial division. J Bacteriol 2008, 190: 2572-2579. 10.1128/JB.01248-07
Chouari R, Le Paslier D, Dauga C, Daegelen P, Weissenbach J, Sghir A: Novel major bacterial candidate division within a municipal anaerobic sludge digester. Environ Microbiol 2005, 7: 1104-1115. 10.1111/j.1462-2920.2005.00795.x
Deppenmeier U, Müller V: Life close to the thermodynamic limit: how methanogenic Archaea conserve energy. Results and problems in cell differentiation 2008, 45: 123-152. 10.1007/400_2006_026
Thauer R, Kaster A, Seedorf H, Buckel W, Hedderich R: Methanogenic Archaea: ecological relevant differences in energy conservation. Nature Rev Microbiol 2008, 6: 579-589. 10.1038/nrmicro1931
Oelgeschläger E, Rother M: Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch Microbiol 2008, 190: 257-269. 10.1007/s00203-008-0382-6
Deppenmeier U, Müller V, Gottschalk G: Pathways of energy conservation in methanogenic archaea. Arch Microbiol 1996, 165: 149-163. 10.1007/BF01692856
Ferry JG: Enzymology of the fermentation of acatate to methane by Methanosarcina thermophila. Biofactors 1997, 6: 25-35. 10.1002/biof.5520060104
Grahame DA: Catalysis of acetyl-CoA cleavage and tetrahydrosarcinapterin methylation by a carbon monoxide dehydrogenase-corrinoid enzyme complex. J Biol Chem 1991, 266: 22227-22233.
Fischer R, Thauer RK: Ferredoxin-dependent methane formation from acetate in cell extracts of Methanosarcina barkeri (strain MS). FEBS Lett 1990, 269: 368-372. 10.1016/0014-5793(90)81195-T
Anderson IJ, Sieprawska-Lupa M, Lapidus A, Nolan M, Copeland M, Del- Rio TG, Tice H, Dalin E, Barry K, Saunders E, Han C, Brettin T, Detter JC, Bruce D, Mikhailova N, Pitluck S, Hauser L, Land M, Lucas S, Richardson P, Whitman WB, Kyrpides NC: Complete genome sequence of Methanoculleus marisnigri Romesser et al. 1981 type strain JR1. Standards in Genomic Sciences 2009., 189:
Nettmann E, Bergmann I, Pramschüfer S, Mundt K, Plagsties V, Hermann C, Klocke M: Polyphasic analysis of methanogenic archaeal communities in agricultural biogas plants. Appl Environ Microbiol 2010, 76: 2540-2548. 10.1128/AEM.01423-09
Ziganshin AM, Schmidt T, Scholwin F, II’inskaja ON, Harms H, Kleinsteuber S: Bacteria and archaea involved in anaerobic digestion of distillers grains with solubles. Appl Microbiol Biotechnol 2011, 89: 2039-2052. 10.1007/s00253-010-2981-9
Anderson I, Ulrich LE, Lupa B, Susanti D, Porat I, Hooper SD, Lykidis A, Sieprawska-Lupa M, Dharmarajan L, Goltsman E, Lapidus A, Saunders E, Han C, Land M, Lucas S, Mukhopadhyay B, Whitman WB, Woese C, Bristow J, Kyrpides N: Genomic characterization of methanomicrobiales reveals three classes of methanogens. PLoS One 2009, 4: 5797. 10.1371/journal.pone.0005797
Southam G, Kalmakoff ML, Jamell KF, Koval SF, Beveridge TJ: Isolation, characterization and cellular insertion of the flagella from two strains of the archaebacterium Methanospirillum hungatei. J Bacteriol 1990, 172: 3221-3228.
Cabillo-Quiroz H, Yavitt JB, Zinder SH: Methanosphaerula palustris gen nov sp nov., a hydrogenotrophic methanogen isolated from a minerotrophic fen peatland. IJSEM 2009, 59: 928-935.
Bräuer SL, Cabillo-Quiroz H, Ward RJ, Yavitt JB, Zinder SH: Methanoregula boonei gen. nov. sp., an acidophilic methanogen isolated from an acidic peat bog. IJSEM 2010, 61: 45-52.
Zhao Y, Boone DR, Mah RA, Boone JE, Xun L: Isolation and characterization of Methanocorpusculum labreanum sp. Nov. from LaBrea tar pits. IJSEM 1989, 39: 10-13.
Brambilla E, Djao ODN, Daligault H, Lapidus A, Lucas S, Hammon N, Nolan M, Tice H, Cheng J-F, Han C, Tapia R, Goodvin L, Putluck S, Liolios K, Ivanova N, Mavromatis K, Mikhailova M, Pati A, Chen A, Palaniappan K, Land M, Hauser L, Chang Y-J, Jeffries CD, Rohde M, Spring S, Sikorski J, Göker M, Woyke T, et al.: Complete genome sequence of Methanoplanus petrolearius type strain (SEBR 4847T). SIGS 2010, 3: 203-211.
Kessler PS, Blank C, Leigh JA: The nif gene operon of the methanogenic archaeon Methanococcus maripaludis. J Bacteriol 1998, 180: 1504-1511.
Galagan JE, Nusbaum C, Roy A, Endrizzi MG, McDolnald P, FritzHugh W, Calvo S, Engels R, Smirnov S, Atnoor D, Brown A, Allen N, Naylor J, Sthange-Thomann N, DeArrellano K, Johnson R, Linton L, McEwan P, McKernan K, Talamas J, Tirrel A, Ye W, Zimmer A, Barber RD, Cann I, Graham DE, Grahame DA, Guss AM, Hedderich R, Ingram-Smith C, et al.: The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res 2002, 12: 532-542. 10.1101/gr.223902
Ercel C, Kube M, Reinhardt R, Liesack W: Genome of Rice Cluster I archaea – the key methane producers in the rice rhizosphere. Science 2006, 313: 370-372. 10.1126/science.1127062
Schnürer A, Houwen FH, Svensson BH: Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch Microbiol 1994, 162: 70-74. 10.1007/BF00264375
Schnürer A, Schink B, Svensson BH: Clostridium ultunense sp. nov., a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogenic bacterium. IJSEM 1996, 46: 1145-1152.
Chouquet CG, Sprott GD: Metal chelate affinity chromatography for the purification of the F420-reducing (Ni, Fe) hydrogenase of Methanospirillum hungatei. J Microbiol Methods 1991, 13: 161-169. 10.1016/0167-7012(91)90016-J
Löffler FE, Sanford RA: Analysis of trace hydrogen metabolism. Environ Microbiol 2005, 397: 22-237.
Thauer RK, Hedderich R, Fischer R: Reactions and enzymes involved in methanogenesis from CO2 and H2. In . Edited by: Ferry JG. Chapman and Hall, New York, London; 1993:209-252. Methanogenesis
Ferry JG: Formate dehydrogenase. FEMS Microbiol Rev 1990, 87: 377-382. 10.1111/j.1574-6968.1990.tb04940.x
Jaenicke S, Zakrzewski M, Jünemann S, Pühler A, Groesmann A, Schlüter A: Analysis of the metagenome from biogas-producing microbial community by means of bioinformatics methods. In Handbook of Molecular Microbial Ecology, vol. II. Metagenomics in Different Habitats. 1st edition. Edited by: Bruijn FJ. Wiley-Blackwell, Hoboken; 2011:403-414.
Herbel Zs, Rákhely G, Bagi Z, Ivanova G, Ács N, Kovács E, Kovács KL: Exploitation of the extremely thermophilic Caldicellulosiruptor saccharolyticus in hydrogen and biogas production from biomasses. Environ Technol 2010, 31: 1017-1024. 10.1080/09593330.2010.484075
Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H: Fluorescence in situ hybridization using 16 S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol 1999, 65: 1280-1288.
McMahon KD, Stroot PG, Mackie RI, Raskin L: Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions – II: Microbial population dynamics. Water Res 2001, 35: 1817-1827. 10.1016/S0043-1354(00)00438-3
Karakashev D, Batstone JD, Angelidaki I: Influence of environmental conditions in anaerobic biogas reactors. Appl Environ Microbiol 2004, 71: 331-338.
Karakashev D, Batstone DJ, Trably E, Angelidaki I: Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl Environ Microbiol 2006, 72: 5138-5141. 10.1128/AEM.00489-06
: Department of Biotechnology. [http://biotech.szbk.u-szeged.hu/index_hun.html]
Miller DN, Byrant JE, Madsen EL, Ghiorse WC: Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol 1999, 65: 4715-4724.
Entcheva P, Liebl W, Johann A, Hartsch T, Streit WR: Direct cloning from enrichment cultures, a reliable strategy for isolation of complete operons and genes from microbial consortia. Appl Environ Microbiol 2001, 67: 89-99. 10.1128/AEM.67.1.89-99.2001
Minas K, McEwan NR, Newbold CJ, Scott KP: Optimization of high throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol Lett 2011, 325: 162-169. 10.1111/j.1574-6968.2011.02424.x
Sambrook J: Fritsch EF, Maniatis T: Molecular cloning: A laboratory manual (second edition). Cold Spring Harbor Laboratory Press, New York; 1989.
Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A, Wilkening J, Edwards RA: The metagenomics RAST server – a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 2008, 9: 386. 10.1186/1471-2105-9-386
Altschul SFl, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997, 25: 3389-3402. 10.1093/nar/25.17.3389
Overbeek R, Begley T, Butler RM, Choudhuri JV, Diaz N, Chuang H-Y, Cohoon M, de Crécy-Lagard V, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi M, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H: The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 2005, 33: 5691-5702. 10.1093/nar/gki866
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevil D, Hu P, Andersen GL: Greengenes, a chimera-checked 16 S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006, 72: 5069-5072. 10.1128/AEM.03006-05
Cole JR, Chai B, Farris RJ, Wang Q, Kulam-Syed-Mohideen AS, McGarrell DM, Bandela AM, Cardenas E, Garrity GM, Tiedje JM: The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 2007, 35: D169-172. 10.1093/nar/gkl889
Wuyts J, Peer Y, Winkelmans T, De Wachter R: The European database on small subunit ribosomal RNA. Nucleic Acids Res 2002, 30: 183-185. 10.1093/nar/30.1.183
Meyer F, Overbeek R, Rodriquez A: FIGfams: yet another set of protein families. Nucleic Acids Res 2009, 37: 6643-6654. 10.1093/nar/gkp698
M5nr non redundant protein database. [http://blog.metagenomics.anl.gov/howto/m5nr-%E2%80%94-the-m5-non-redundant-protein-database/]
Acknowledgments
This work was supported by EU projects HUSRB/1002/214/041 IPA and HURO/1001/193/2.2.2. CBC. Domestic funds from GOP-1.1.2.-07/1-2003/8-0007, TÁMOP-4.2.1/B-09/1/KONV-2010-0005, Baross_DA07_DA_TECH-07-2008-0012, Baross_ALGOLABH, OMFB-00356/2010 and TÁMOP-4.2.2/B-10/1-2010-0012 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
RW developed the DNA extraction protocol and participated in the evaluation of the data. EK carried out the anaerobic digestion experiments and supervised the operation of the fermenters. GM organized and performed the next-generation sequencing and took part in the data analysis. BZ participated in the experimental work and its design. GR contributed to the data interpretation. KLK conceived the study, participated in its design and compiled the manuscript. All the authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wirth, R., Kovács, E., Maróti, G. et al. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol Biofuels 5, 41 (2012). https://doi.org/10.1186/1754-6834-5-41
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1754-6834-5-41