Abstract
Background
Yeast is considered to be a workhorse of the biotechnology industry for the production of many value-added chemicals, alcoholic beverages and biofuels. Optimization of the fermentation is a challenging task that greatly benefits from dynamic models able to accurately describe and predict the fermentation profile and resulting products under different genetic and environmental conditions. In this article, we developed and validated a genome-scale dynamic flux balance model, using experimentally determined kinetic constraints.
Results
Appropriate equations for maintenance, biomass composition, anaerobic metabolism and nutrient uptake are key to improve model performance, especially for predicting glycerol and ethanol synthesis. Prediction profiles of synthesis and consumption of the main metabolites involved in alcoholic fermentation closely agreed with experimental data obtained from numerous lab and industrial fermentations under different environmental conditions. Finally, fermentation simulations of genetically engineered yeasts closely reproduced previously reported experimental results regarding final concentrations of the main fermentation products such as ethanol and glycerol.
Conclusion
A useful tool to describe, understand and predict metabolite production in batch yeast cultures was developed. The resulting model, if used wisely, could help to search for new metabolic engineering strategies to manage ethanol content in batch fermentations.
Similar content being viewed by others
Background
Management of ethanol yields is emerging as one of the most relevant challenges for biotechnology, including both ethanol maximization (i.e. bioethanol and distilled beverages industries) and reduction/minimization (i.e. wine, bakery and commodities industries). Significant advances have been made in modeling ethanol fermentations [1–5], although steady-state, gene-modification strategies have resulted in varying degrees of success (for review see [6–8]) mainly due to growth impairment. In turn, dynamic models are able to describe and predict batch fermentations' time-courses better, since different metabolic stages are considered.
A widely used modeling approach to predict cell behavior beyond calibration data is Flux Balance Analysis (FBA), which represents cell biochemical networks as a set of underdetermined constrained mass-balances. In this framework, linear programming is applied to generate a flux distribution that optimizes a given objective function, subject to flux balance equations and constraints. Objective functions commonly used are maximization of ATP production [9, 10], maximization of biomass formation [11–13], minimization of metabolic adjustment (MoMa) [14] or minimization of ATP consumption [15, 16]. So far, growth maximization has been the most extensively used approach to describe the physiology during growth.
Applying FBA in large-scale metabolic reconstructions, termed Genome-Scale metabolic models (GSMM), has allowed establishing a direct relationship between genetic data and metabolic activity fluxes. These models have been shown to be very useful in predicting the physiological behavior of a microorganism under different genetic and environmental disturbances, i.e. growth rate and product secretion patterns [11, 13, 17–19]. GSMM can help to speed up the design of cells with improved and desired properties, providing metabolic engineering targets that are experimentally testable [20–22]. For example, bacterial genome-scale models have been used to design strains that overproduce lycopene [23], lactate [24], succinate [25], 1,3-propanediol [26], hydrogen [20], L-valine [27] and L-threonine [28]. In turn, genome-scale yeast models have been mainly applied to design ethanol overproducing strains [22, 29–31]; nevertheless, design of ethanol underproducing strains have been overlooked, a key issue in wine industry today [5, 32–34].
Many computational tools for identifying strain modifications leading to targeted overproductions have been described in the literature. One of the earliest efforts was the OptKnock [26] procedure that proposed gene knockouts leading to targeted overproductions. Later, OptReg [35] expanded OptKnock to consider not only knockouts but also overexpressions and down regulations. In addition, OptStrain [20] allowed for knock-ins of non-native genes to enable production of desired biochemicals. Evolutionary search procedures for solving the resulting combinatorial optimization problems were explored in OptGene [36]. Recently, OptForce [21] was used to identify flux manipulation leading to targeted overproductions. However, strain optimization requires taking into account the whole bioprocess, i.e. growth as well as non-growth periods, such as lag, log and steady-state phases. Furthermore, strain optimization needs microbial metabolic modeling expanded upon constraint-based FBA that incorporates experimental validation [37], which has not always been considered in GSMM development. Including these issues will improve the capability of the models to predict the impact of several environmental and genomic alterations on the course of batch fermentations.
Several yeast genome-scale metabolic models have been described so far: iFF708, iND750, iLL672, iIN800, iMM904 and Yeast 4.0 [18, 19, 38–41]. Despite their advantages, all of them can only simulate time-invariant extracellular conditions consistent with continuous culture, but are not able to reproduce features of the microbial growth process. Dynamic FBA models are increasingly applied to simulate bacteria [42–45], yeast [15, 46, 47], plant [48] and animal [49, 50] growth under several conditions [51, 52]. However, the application of dynamic genome-scale FBA models for yeast [29, 30] and bacteria [52–54] has been barely explored, and only one bacterial dynamic GSMM model has been experimentally validated.
Over the past few years, our group has been developing a dynamic FBA model (DFBA) to represent anaerobic S. cerevisiae batch fermentations [15, 47]. This model, comprising a reduced stoichiometric network (39 metabolic reactions), accurately described fermentation profiles under different environmental conditions. However, the lack of detailed metabolic description makes it unsuitable for metabolic engineering studies. To overcome this limitation, we expanded our previous DFBA model of S. cerevisiae metabolism [15, 47] to genome-scale. This model included kinetic expressions to dynamically constrain the uptake of nutrients, biomass and maintenance. In addition, key improvements related to anaerobic metabolism were identified. Furthermore, we carried out a comprehensive validation using experimental profiles of batch fermentations and final concentration of metabolic products under genetic/environmental disturbances. To the best of our knowledge, we report here the first experimentally validated GS-DFBA for S. cerevisiae.
Methods
Model formulation
The current version of our genome-scale dynamic-flux-balance-analysis model (GS-DFBA) consists of four interacting blocks, which are solved sequentially (Figure 1). First, initial conditions and fixed constraints are specified (block 0). Next, dynamic constraints which depend on metabolite concentrations in the medium are established (block 1). A linear programming (LP) problem is then solved (block 2) to compute the consumption and production rates of extracellular metabolites. Finally, these production rates are transferred to an ODE solver (block 3) that integrates the respective bioreactor mass balances during intervals of 30 minutes (keeping extracellular rates constant), providing accurate results without losing fermentation details [15, 47]. A sensitivity analysis showed small variations when this time interval is changed (Additional file 1). The procedure iterates between blocks 1, 2 and 3 until the sugar is consumed or an unfeasible condition is found.
Resolution algorithm of idFV715 model. The model is based on an iterative optimization of an under determined matrix, using LINDO optimization software (LINDO system): 0) Fixed constraints to be used throughout fermentation are defined, such as genetic background or nutritional requirements; 1) Dynamic constraints are defined as bounds fluxes set to by intracellular extracellular conditions; 2) LP solves the metabolic flux distribution, as well as the consumption and production rates, at 30 min intervals; 3) The resulting rates are used as inputs for the differential equations solved using a variable step integration routine.
Typical initial conditions that must be defined a priori are temperature, and the concentration of nitrogen-compounds, sugar-compounds, extracellular metabolites and biomass. The LP is bounded by fixed and dynamic constraints. Fixed constraints include carbon/nitrogen limiting medium, oxygen presence or absence, biomass equation, and genetic background. Dynamic constraints include sugar/nitrogen uptake kinetics, maintenance and carbohydrate accumulation. Following this procedure, it takes 43 s of CPU time to simulate a normal alcoholic fermentation (200 g/L of sugars and 300 mg/L of assimilable nitrogen) in a Core2Duo 2.66 GHz processor. The features of each block are detailed below (Figure 1).
Linear programming
A standard FBA model [13, 55, 56] comprises an underdetermined metabolic network of s molecular species and v reactions, which is represented by a v × s stoichiometric matrix (T). To solve the intracellular fluxes as well as the consumption and production rates of metabolites in the cell, an LP is defined (eq. 1), where an objective function for cell metabolism vj should be specified. Following common practice, maximization of growth rate was used to mimic growth in exponential phase [11–13]; however, when the limiting nutrient is exhausted, minimization of ATP consumption by maintenance was applied [15, 16].

where v is the vector of metabolic fluxes, and  and
and  are dynamic lower and upper bounds of the flux i, respectively. All LP calculations were carried out within MATLAB (The MathWorks Inc.) using Lindo optimization package (Lindo Systems Inc.).
are dynamic lower and upper bounds of the flux i, respectively. All LP calculations were carried out within MATLAB (The MathWorks Inc.) using Lindo optimization package (Lindo Systems Inc.).
To reduce the impact of multiple optimal solutions that normally appear in FBA [57], for metabolic engineering approaches we carried out a bi-level optimization procedure [29], solving first the LP for maximum biomass, and then fixing the biomass at this maximum value and solving the LP again to optimize a second objective (i.e. maximizing/minimizing ethanol).
Up until now, several yeast genome-scale model databases have been published: iFF708 [18], iND750 [19], iLL672 [40], iIN800 [39], iMM904 [38] and Yeast 4.0 [41]; each one adding more details to the metabolic network. However, additional information regarding compartmentalization, cycles and expansion of previously lumped reactions has not necessarily led to improved accuracy and better predictions.
The first published genome-scale model, iFF708, was the simplest and has good predictive performance. Therefore, The GS-DFBA model described here was developed using an updated version of the iFF708 stoichiometric matrix [18] coupled with our previously developed algorithms of DFBA (Figure 1) [15, 47]. The resulting model, idFV715, where "d" stands for dynamic and "i" for in silico, consists of 715 structural ORF's that catalyze 705 distinct biochemical reactions and 145 putative reactions that have not been associated with any gene yet, comprising 590 metabolites and 1181 metabolic reactions in total. Compared with iFF708, the idFV715 model includes a more detailed description of flavor production related to acetoin and butanediol syntheses.
Dynamic mass balance
During microbial growth, the environmental conditions are changing constantly due to the consumption of nutrients and secretion of metabolites. These changes are modeled by the following set of ordinary differential equations:

Where M EX is the vector of concentrations of extracellular metabolites in the medium, V EX is the vector of the respective specific consumption and production rates, and X V is the concentration of viable biomass in the medium.
Fixed constraints
These include all constraints that remain constant during the whole fermentation, such as genetic background (deletions, insertions and overexpressions), carbon/nitrogen limiting medium that modify the biomass equation, and lack of oxygen that modify oxygen-related reactions.
As yeast responds differently to aerobic or anaerobic conditions [58–60] we described the S. cerevisiae physiology under these conditions as closely as possible. The expression of approximately one hundred genes is regulated by oxygen availability [61, 62], affecting mainly the cell redox state [63]. This, in turn, affects ATP, biomass and product syntheses. For instance, approximately eight times less ATP and ten times less biomass are produced under anaerobic conditions [64, 65].
In our approach, we bounded to zero the oxygen uptake-related flux. In addition, we included a known transcriptional regulation, i.e. during anaerobic fermentation the TCA cycle has been shown to be broken at the level of succinyl CoA synthetase, acting as two separate branches - the "oxidative branch" and the "reductive branch." This behavior was first established as thermodynamically feasible in S. cerevisiae [66], and then supported by Metabolic Flux Analysis (MFA) [67]. Later, Camarasa et al. [68] experimentally confirmed it, using NMR isotopic filiation. Hence, the genes involved in succinate dehydrogenase complex and succinyl-CoA ligase were bound to zero, thus forcing the TCA cycle to act as two branches. Also, quinone mediated reactions involving FADH2 and NADH reoxidation were turned off. Finally, ergosterol, lanosterol and zymosterol upper bound constraints, UB, were set to infinity (assumed to be non-limiting) in anaerobic conditions [69–71].
Furthermore, to ensure the presence of inorganic compounds in every condition studied, phosphate and sulphate concentration in the medium were also set to infinity (UB assumed to be non-limiting).
Dynamic constraints
Sugar transport is the main rate-limiting step in N-limited fermentations [72]. Known regulatory effects have been previously used by our group to include sugar uptake expressions into a DFBA model [1]. The efficiency of the uptake of sugars is altered by the gene expression, where a set of specific hexose transporters (HXT) are expressed in response to environmental signals [73, 74]. Moreover, these transporters show competitive inhibition between glucose and fructose, and their activity is modulated by temperature through changes in the activation energy of the process as well as by non-competitive inhibition by ethanol [75]. Kinetic expressions that considered all these effects were incorporated as UB (upper bound) into the idFV715 model (Additional file 2: eqs. 1, 2).
An empirical function was developed to describe total YAN (Y east A ssimilable N itrogen) consumption. We incorporated normalized time-varying fluxes for each nitrogen-compound (amino acids/ammonia) into a previously reported equation for total nitrogen consumption [15, 47], which was derived from an experimental lookup-table using TableCurve 2D software (Systat Software Inc.). The uptake flux of each N-compound changes according to total nitrogen concentration in the medium, representing a competition for the limited number of nitrogen transporters. These fluxes were included as UB constraints in the LP (Additional files 2: eq. 3).
Yeast faces changing conditions during fermentation, triggering metabolic stress responses and increasing ATP consumption by maintenance (mATP) [34, 76, 77], as determined in media with ethanol concentrations above 4% v/v [78]. High temperatures also result in increased cellular maintenance, to restore the membrane potential lost by ion diffusion and from altered protein synthesis [79, 80]. In addition, sluggish fermentations arising from nitrogen deficiency show higher maintenance costs than normal fermentations [81]. Therefore, to adequately model yeast response to stress, a maintenance term was included that estimate the specific energy requirements to maintain cellular function during fermentation. A lower bound to mATP consumption is set by an empirical function that assigns maintenance costs according to fermentation conditions [47] (Additional file 2: eq. 4). This function, fitted from an experimental lookup-table using TableCurve 2D software, represents the influence of ethanol, high temperature and other energy expenditures associated with sluggish cultures [15].
Accurate simulation of the growth rate in FBA models requires a careful formulation of the biomass equation [38–40, 82]. This should reflect observations that biomass composition changes with environmental conditions and as fermentation progresses [39, 81, 82]. Hence, we used a biomass equation that considered new data and experimentally observed variations in the biomass composition of S. cerevisiae under different growth conditions. This medium-specific biomass equation was reported in iIN800 model [39] (Additional file 2). Our approach incorporates an empirical carbohydrates accumulation expression (Additional file 2: eq. 5)[15] to the medium-specific biomass equation [39] to give time-dependent behavior. The equation describing the accumulation of carbohydrates was determined by fitting measured rates to sugar consumption [15]. This function defines an UB in the LP. This final equation is referred to in this article as time-medium-specific biomass equation.
Model validation
Experimental fermentations
idFV715 was validated with data obtained in the laboratory as well as from industrial fermentations. Laboratory data comprised 12 fermentations carried out by our group [15, 79] and 8 fermentations provided by the Sciences pour l'Oenologie Research Unit, INRA, Montpellier, France (Drs. Sylvie Dequin and Carole Camarasa, unpublished). Both datasets correspond to alcoholic fermentations performed at several temperatures (20-34°C) under different initial carbon (150-308 g sugar/L) and nitrogen (50-538 mg N/L) concentrations [15, 79]. All were carried out in 1 L bioreactors using S. cerevisiae EC1118 or V5 wine strains.
Industrial data come from 10 anisothermic wine fermentations with different initial sugar concentrations (181-250 g/L) [79]. These were performed during the 2003 vintage in Chile; in fermentation tanks of 40 to 60 m3 of Cabernet Sauvignon inoculated with S. cerevisiae EC1118 strain.
In silico genetic modifications
The impact of the gene modifications on simulations of batch fermentations, as measured in final product concentrations, was compared with literature data. To simulate the effect of a single gene deletion, the fluxes through the corresponding reactions were constrained to zero during the whole fermentation [13, 42]. In silico detrimental gene deletions lead to lower growth rates compared to wild-type simulations, yielding lower biomass and/or longer fermentation times.
We assessed the predictive performance of the idFV715 model by comparing simulation results with 35 different environmental and genetic modifications reported in the literature. These include overexpressions [5, 83–85], deletions [5, 83, 84, 86–89], insertions [90] and cofactor engineering [5] in single, double and triple mutant strains (Additional file 3). The "R" score was used to assess model performance:

This expression compares experiments and simulations in terms of the relative changes in the final concentration of given metabolites. Here, D is the modeling (M) or experimental (E) concentration of the metabolite "i" when it is genetically modified (GM) or wild type (WT). Hence, values of R close to zero mean good model predictions.
Fermentation conditions that were not given in the literature were estimated to reproduce the respective experimental results as closely as possible. For example, environmental conditions such as temperature, oxygen, sugars, amino acids, ammonia, ethanol and glycerol, were normally given in the literature, but vitamins and inorganic compounds of the medium were not. In addition, deletions and insertions were well described in the literature references; however, the levels of overexpression were not given. Therefore, due to the nature and diversity of information sources, estimating fermentation conditions was often difficult and an additional source of error.
Results and discussion
Expanding DFBA to genome-scale
In this study, we expanded a small and reliable 39-equation DFBA model of S. cerevisiae metabolism [15, 47] to genome-scale. Our aim was to obtain increased insight into yeast metabolism (given by the genome-scale), while maintaining simulation performance of the previous DFBA model. Therefore, we built a GS-DFBA (idFV715) that accurately predicts the synthesis and consumption profiles of yeast metabolites during the alcoholic fermentation.
To assess the new model, first we analyzed the impact on simulation performance of key model improvements. Then, we validated model results with experimental data.
Assessment of model improvements
Proper choice of the biomass equation is normally considered a critical component in FBA-based modeling [38–40, 82], although adequate handling of ATP maintenance and anaerobic conditions are also key issues that have been overlooked so far. In this study, we explore the impact on the idFV715 model's predictions of maintenance, biomass and anaerobic constraints.
Maintenance
Fermentations were simulated anaerobically in N-limiting medium; the variables were low and high temperatures, as well as sluggish and normal conditions. In each case, four maintenance models were assessed: i) mATP constrained according to eq. (4) (Additional file 2), ii) mATP constrained to zero, iii) unconstrained mATP, and iv) mATP constrained to a typical value used in previous models (see Table 1).
The most significant difference among model simulations was glycerol prediction. When maintenance flux is constrained according to our maintenance equation (Additional file 2: eq. 4), glycerol predictions are much closer to measured values than the other simulations. In addition, simulations with unbounded or zero maintenance tend to overpredict biomass concentrations, partially explaining the impaired results on glycerol synthesis obtained in these simulations. This is probably due to new environmental conditions where maximization of the growth rate resulted in minimizing ATP costs from glycerol synthesis. Furthermore, when simulations were constrained to a commonly used value from previous genome-scale models (i.e. 1 mmol/g DW h-1) [18, 63], the ATP maintenance expenditure is fixed with a higher value than the output of the maintenance equation proposed in this paper. This higher maintenance cost results in lower biomass and glycerol synthesis and hence in longer fermentation times.
Ethanol prediction also showed significant differences among models. A decrease in glycerol synthesis results in increased carbon fluxes through glycolysis. Hence, unbounded, zero maintenance and constrained-to-previous-value simulations result in higher ethanol contents than those obtained with our maintenance equation (Additional file 2: eq. 4).
Moreover, the latter predicts ethanol concentrations closer to experimental data. The model with our maintenance equation tends to overestimate ethanol synthesis in the nitrogen-lean condition though. To grow under stress, yeasts might divert the carbon flux to other metabolic pathways not accounted for in our model, such as synthesis of lipids and cell-wall precursors. Therefore, a careful examination of these components could be useful to improve model results.
Biomass composition
Since biomass composition varies throughout fermentation and depends on media composition, a time-medium-specific biomass expression was included (see methods and Additional file 2). To assess the impact on model predictions of this biomass expression, two simulations were carried out using i) current biomass expression; and ii) iFF708 biomass expression (Table 2).
The updated time-dependent biomass expression developed in this work significantly improved biomass predictions compared to simulations using the iFF708 biomass expression. In the first case, using experimental data, an average error ranging from 0.2-0.6 g/L was found. In turn, iFF708 biomass calculations with the GS-DFBA showed deviations between 0.7-1.4 g/L. In both cases, better performances were accomplished in fermentations with standard nitrogen levels (300 mg/L YAN), compared to lean-nitrogen cultures. Underestimation of cell growth under low-nitrogen conditions strongly suggests that cells maximize the use of nitrogen compounds under nutrient limitation, a fact that is not accounted in idFV715.
Anaerobic conditions
To assess the impact of anaerobic constraints on model predictions, we performed simulations by varying the activation or inhibition of the following specific constraints: i) oxygen uptake constrained to zero, ii) unconstrained sterol and unsatured fatty acids uptake, iii) TCA cycle split up into two branches, and iv) quinone-mediated reactions constrained to zero (Table 3).
Simulations in the absence of oxygen were only feasible when the availability of unsaturated fatty acids and sterols is not constrained (Table 3: model 6). Since S. cerevisiae cannot synthesize these compounds (ergosterol and zymosterol) under anaerobic conditions [69–71], this constraint is usually considered in anaerobic models [29, 30].
However, restrictions to TCA and quinone-reactions have received little attention and few studies have incorporated transcriptional regulatory information to respond to these environmental conditions [91]. We found that TCA constraints are essential to describe the two separate branches operating under anaerobic conditions - the "oxidative branch" and the "reductive branch" [66–68], which are critical to get accurate predictions. In this condition, glycerol and ethanol synthesis compared very well with experimental data (Table 3: models 1, 3 and 4). When the TCA cycle is unconstrained, glycerol synthesis decreases and ethanol increases. This is probably due to the TCA cycle working as a reducing cycle under these unconstrained conditions, reducing the excess NADH. Consequently, glycerol synthesis would be no longer needed, increasing carbon flux through glycolysis and so, ethanol synthesis.
In addition, results showed that quinone-mediated oxidations of FADH2 and NADH play a fundamental role in aerobiosis (not shown); however, under anaerobiosis their effect worsens model predictions. When these reactions are turned off, predictions of fermentation time, biomass, glycerol, and ethanol synthesis are closer to measured values (Table 3: models 2, 4). This is because in idFV715, quinone-mediated reactions do not require oxygen as a precursor, since they always act as electron acceptors, replacing the redox function of glycerol synthesis. Consequently, the rate of ethanol synthesis increases due to a higher carbon flux in glycolysis.
In summary, a significant improvement in glycerol, ethanol, biomass and fermentation time prediction were achieved when the model included the full set of anaerobic constraints described above, in addition to the new maintenance and biomass equations.
Model validation
We considered for validation 2 types of published experimental data: i) batch fermentation kinetics; and ii) final concentration of metabolic products under genetic/environmental disturbances. These analyses highlight the differences with our previous model while maintaining model performances.
Fermentation profiles
Two sets of batch fermentations with wt yeasts were used. The first set corresponded to 20 laboratory fermentations under different temperature and initial concentrations of nitrogen and sugars. Ten industrial wine fermentations with different initial glucose concentrations and a time varying temperature profile comprised the second set.
Table 4 summarizes performance results for idFV715 validation. The numbers in the respective boxes correspond to the correlation, R, between simulation and experimental data for the whole fermentation process. Figure 2 shows consumption and production profiles for main metabolites and nutrients included in Table 4. Sugar prediction at industrial and laboratory scale shows good agreement with experimental data at several initial sugar conditions (Figure 2C, D). Furthermore, sugar prediction shows good correlation at high-nitrogen and low-nitrogen conditions, reflecting standard and sluggish fermentations (Figure 2E). Therefore, for laboratory and industrial fermentations, idFV715 shows a high degree of confidence in sugar uptake profile predictions (glucose and fructose). This step ensures the correct income of energy and carbon to the modeled cell.
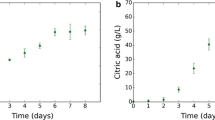
idFV715 model performance. Model predictions of consumption and production rates of the main metabolites and nutrients involved in an alcoholic fermentation. In this figure, symbols represent the experimental data and lines represent model prediction. Measured values were in triplicate with a CV <5%: A: Experiments and simulations of isothermal, laboratory-scale fermentations, in 4 conditions of total-assimilable-nitrogen (100, 200, 300, 400 mg/L of YAN); B: Experiments and simulations of isothermal, laboratory-scale, high-nitrogen fermentations (300 mg/L of YAN, R = 99%, 400 points), 28°C; C: Experiments and simulations of isothermal, laboratory-scale, high-nitrogen fermentations. Residual sugar concentration at two representative initial conditions of sugar content in the medium (240 and 182 g/L, 300 mg/L YAN, 28°C); D: Experiments and simulations of anisothermal, industrial-scale, high-nitrogen (240 mg/L YAN) fermentations. The fastest and slowest fermentations are shown. Simulations werefile run assuming a typical temperature profile (dotted-line); E: Isothermal, laboratory-scale, residual sugar concentration for the fastest and the slowest fermentation analyzed, corresponding to 50 mg/L YAN and 300 mg/L, respectively; F: Isothermal, laboratory-scale, predicted concentration of biomass under 50 mg/L (closed circles) YAN and 300 mg/L (×) YAN 28°C; G: Isothermal, laboratory-scale, predicted concentration of ethanol (×) and glycerol (closed circles) under 240 g/L sugar, 300 mg/L YAN, 28°C; H: Isothermal, laboratory-scale, predicted concentration of ethanol (×) and glycerol (closed circles) under 182 g/L sugar, 300 mg/L YAN, 28°C.
Nitrogen is usually the limiting nutrient in alcoholic fermentations and it is linked to the redox-balance that eventually determines the synthesis of glycerol, ethanol and acetate, among other metabolites. Despite its relevance, most current models simply define ammonia uptake as the sole nitrogen source or leave amino acid uptake unbounded; only one bacterial GS-DFBA model applied kinetic parameters to define amino acid uptake, although it has not been experimentally validated [53]. Here, we showed that idFV715 with these new nitrogen features accurately predicts nitrogen uptake experimental profiles, i.e. ammonia and amino acids kinetics, at several initial nitrogen conditions (see Figure 2A, B and Table 4). This feature is useful to define the correct synthesis of redox-linked metabolites.
Simulated profiles of ethanol and glycerol in laboratory-scale fermentations compare well with experimental data (Figure 2G, H). Moreover, prediction of the biomass profile is in relatively close agreement with experimental data; however they are less accurate at low nitrogen conditions (Figure 2F). This could be partly explained by an experimentally observed delay in biomass synthesis in relation to nitrogen uptake that was not captured by idFV715, and also by a lower biomass prediction at these conditions (See section Assessment of model improvements).
Remarkably, when same data sets were used to compare the idFV715 model to our previous reduced DFBA model [15], on average, performances were quite similar (differences in correlation < 5%) (Additional file 3). Both models show high correlation and a high predictive ability (less than 5% of the real value, with 95% of confidence). Hence, good performance was quite maintained after expansion to the genome-scale.
In summary, despite some differences between model and experimental data, idFV715 predicts relatively well the consumption and synthesis profiles of the main metabolites/nutrients involved in an alcoholic fermentation.
Final product concentration
In order to analyze the additional features of the GS-DFBA model, final measured concentrations of metabolic products were compared to idFV715 predictions for 35 fermentations using yeast mutants under a variety of environmental conditions. This set of experiments comprises deletions, overexpressions and insertions in up to three genes (Additional file 3, section 2). These features represent one-step forward compared to our previous model capabilities [15], which had not enough reactions to simulate genetic disturbances.
Analyses of final product concentrations showed that ethanol, glycerol and biomass content - the major carbon compounds in alcoholic fermentation - were predicted in close agreement with experimental data (Figure 3). Indeed, ethanol predictions were accurate, with maximum deviations smaller than 5%. Glycerol and biomass synthesis showed an acceptable level of confidence with errors smaller than 50%. In turn, the model often overestimated acetate production. A sensitivity analysis showed that acetate synthesis in the model is highly sensitive to temperature, nitrogen and sugar (Additional file 1). Hence, small errors in these variables (taken from the respective paper or database) can cause large variations in the predicted final acetate concentration. If, in addition, we consider that acetate synthesis represents less than 0.5% of the initial sugar, it is not surprising that idFV715 does not perform well for acetate prediction. Moreover, synthesis and regulation of acetate are not well known, and FBA models include only carbon and redox governed metabolic rearrangements; transcriptional and protein regulations are not taken into account. In a future version of the model, inclusion of some regulatory network could improve these results as other studies have explored [46, 91, 92].
Prediction error distribution for idFV715. Prediction error distribution for 35 different yeast fermentations. The errors compare the relative change between model performance and experimental results, under different environmental conditions (considering 95% confidence). Mean (●)and median value of each set (▲).
Instead of predicting exact final concentration values, the model is able to reproduce the relative effects and trend of given genetic engineering strategies on final metabolites concentrations. For example, our model reproduced the experimental results of Guadalupe et al. (2010) [90]. These authors expressed the E. coli MHPF gene in S. cerevisiae, encoding the NAD-dependent acetaldehyde dehydrogenase to restore redox balance and allow the anaerobic growth of a S. cerevisiae Δgpd1/gpd2 strain through acetate consumption. Including these gene modifications, our model predicted an increment of ethanol production of 5%, no glycerol production and consumption of acetate instead of production. Although the model predicts the right trend, the experimental values obtained by Guadalupe et al. (2010) regarding ethanol and acetate were different; they obtained twice the amount of ethanol and almost 7 times more acetate was consumed.
Conclusions
In this paper, we described the first experimentally validated GS-DFBA model for alcoholic fermentations. Model validation included fermentation profiles and final concentration of fermentation products under different environmental and/or genetic disturbances. We implemented a set of constraints that resulted in key improvements in the physiological response of S. cerevisiae under anaerobic fermentation. Here, we showed that not only biomass expression is a critical component of FBA-based modeling but also ATP maintenance and anaerobic constraints. Furthermore, it is noteworthy that a detailed description of nitrogen-compounds uptake helps to improve results. Therefore, under the conditions studied here, idFV715 closely agrees with final concentrations as well as fermentation profiles of the main metabolites involved in alcoholic fermentation, especially ethanol, glycerol and biomass. Minor compounds such as acetate are not well predicted though, mainly due to unaccounted genetic regulations and model sensitivity. A careful examination in the definition of lipids and cell-wall components could help to improve results.
Consequently, idFV715 could be useful to predict the evolution and concentration of the main metabolites from S. cerevisiae under different environmental conditions as well as different genetic backgrounds. Given the close predictions of idFV715 regarding ethanol synthesis, this model is especially suitable to design yeast strains for optimum ethanol management in batch fermentations. This model and its future improvements can be used to design new metabolic engineering strategies to optimize fermentation, as well as product synthesis.
Abbreviations
- DFBA:
-
Dynamic Flux Balance Analysis
- GSMM:
-
Genome-Scale Metabolic Model
- GS-DFBA:
-
Genome-Scale, Dynamic Flux Balance Analysis
- MFA:
-
Metabolic Flux Analysis
- MoMA:
-
Minimization of Metabolic Adjustments
- NMR:
-
Nuclear Magnetic Resonance
- LP:
-
Linear Programming
- ODE:
-
Ordinary Differential Equation
- TCA:
-
Tricarboxylic acid
- YAN:
-
Yeast Assimilable Nitrogen.
References
Kong QX, Cao LM, Zhang AL, Chen X: Overexpressing GLT1 in gpd1 mutant to improve the production of ethanol of Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2007, 73: 1382-1386. 10.1007/s00253-006-0610-4
Kong QX, Gu JG, Cao LM, Zhang AL, Chen X, Zhao XM: Improved production of ethanol by deleting FPS1 and over-expressing GLT1 in Saccharomyces cerevisiae. Biotechnol Lett. 2006, 28: 2033-2038. 10.1007/s10529-006-9185-5
Valadi H, Larsson C, Gustafsson L: Improved ethanol production by glycerol-3-phosphate dehydrogenase mutants of Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1998, 50: 434-439. 10.1007/s002530051317
Seo HB, Kim SS, Lee HY, Jung KH: High-level production of ethanol during fed-batch ethanol fermentation with a controlled aeration rate and non-sterile glucose powder feeding of Saccharomyces cerevisiae. Biotechnol Biop Eng. 2009, 14: 591-598. 10.1007/s12257-008-0274-2.
Ehsani M, Fernandez MR, Biosca JA, Julien A, Dequin S: Engineering 2, 3-butanediol dehydrogenase to reduce acetoin formation by glycerol overproducing low-alcohol wine yeast. Appl Environ Microbiol. 2009, 75: 3196-3205. 10.1128/AEM.02157-08
Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF: Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol. 2007, 74: 937-953. 10.1007/s00253-006-0827-2
Nevoigt E: Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2008, 72: 379-412. 10.1128/MMBR.00025-07
Matsushika A, Inoue H, Kodaki T, Sawayama S: Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives. Appl Microbiol Biotechnol. 2009, 84: 37-53. 10.1007/s00253-009-2101-x
Ra M, Mm D: Simple constrained optimization view of acetate overflow in E. coli. Biotechnol Bioeng. 1990, 35: 732-738. 10.1002/bit.260350711
Ramakrishna R, Edwards JS, McCulloch A, Palsson BO: Flux-balance analysis of mitochondrial energy metabolism: consequences of systemic stoichiometric constraints. Am J Physiol. 2001, 280: 695-704.
Kauffman KJ, Prakash P, Edwards JS: Advances in flux balance analysis. Curr Opin Biotechnol. 2003, 14: 491-496. 10.1016/j.copbio.2003.08.001
Edwards JS, Palsson BO: The Escherichia coli MG1655 in silico metabolic genotype: its definition, characteristics, and capabilities. Proc Natl Acad Sci USA. 2000, 97: 5528-5533. 10.1073/pnas.97.10.5528
Price ND, Papin JA, Schilling CH, Palsson BO: Genome-scale microbial in silico models: the constraints-based approach. Trends Biotechnol. 2003, 21: 162-169. 10.1016/S0167-7799(03)00030-1
Segre D, Vitkup D, Church GM: Analysis of optimality in natural and perturbed metabolic networks. Proc Natl Acad Sci USA. 2002, 99: 15112-15117. 10.1073/pnas.232349399
Pizarro F, Varela C, Martabit C, Bruno C, Perez-Correa JR, Agosin E: Coupling kinetic expressions and metabolic networks for predicting wine fermentations. Biotechnol Bioeng. 2007, 98: 986-998. 10.1002/bit.21494
Raghunathan AU, Perez-Correa JR, Agosin E, Biegler LT: Parameter estimation in metabolic flux balance models for batch fermentation--Formulation & Solution using Differential Variational Inequalities (DVIs). Ann Op Res. 2006, 148: 251-270. 10.1007/s10479-006-0086-8.
Alper HHS: Development of systematic and combinatorial approaches for the metabolic engineering of microorganisms. PhD Thesis. 2006, MIT, Metabolic engineering department
Forster J, Famili I, Fu P, Palsson BO, Nielsen J: Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res. 2003, 13: 244-253. 10.1101/gr.234503
Duarte NC, Herrgård MJ, Palsson B: Reconstruction and Validation of Saccharomyces cerevisiae iND750, a Fully Compartmentalized Genome-Scale Metabolic Model. Genome Res. 2004, 14: 1298-1309. 10.1101/gr.2250904
Pharkya P, Burgard AP, Maranas CD: OptStrain: a computational framework for redesign of microbial production systems. Genome Res. 2004, 14: 2367-2376. 10.1101/gr.2872004
Ranganathan S, Suthers PF, Maranas CD: OptForce: An Optimization Procedure for Identifying All Genetic Manipulations Leading to Targeted. PLoS Comp Biol. 2010, 6: 1-11.
Bro C, Regenberg B, Förster J, Nielsen J: In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production. Metab Eng. 2006, 8: 102-111. 10.1016/j.ymben.2005.09.007
Alper H, Jin YS, Moxley JF, Stephanopoulos G: Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng. 2005, 7: 155-164. 10.1016/j.ymben.2004.12.003
Fong SS, Burgard AP, Herring CD, Knight EM, Blattner FR, Maranas CD, Palsson BO: In silico design and adaptive evolution of Escherichia coli for production of lactic acid. Biotechnol Bioeng. 2005, 91: 643-648. 10.1002/bit.20542
Wang Q, Chen X, Yang Y, Zhao X: Genome-scale in silico aided metabolic analysis and flux comparisons of Escherichia coli to improve succinate production. Appl Microbiol Biotechnol. 2006, 73: 887-894. 10.1007/s00253-006-0535-y
Burgard AP, Pharkya P, Maranas CD: Optknock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization. Biotechnol Bioeng. 2003, 84: 647-657. 10.1002/bit.10803
Park JH, Lee KH, Kim TY, Lee SY: Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA. 2007, 104: 7797-7802. 10.1073/pnas.0702609104
Lee KH, Park JH, Kim TY, Kim HU, Lee SY: Systems metabolic engineering of Escherichia coli for L-threonine production. Mol Syst Biol. 2007, 3: 1744-4292.
Hjersted JL, Henson MA, Mahadevan R: Genome-scale analysis of Saccharomyces cerevisiae metabolism and ethanol production in fed-batch culture. Biotechnol Bioeng. 2007, 97: 1190-1204. 10.1002/bit.21332
Hjersted JL, Henson MA: Steady-state and dynamic flux balance analysis of ethanol production by Saccharomyces cerevisiae. IET Systems Biology. 2009, 3: 167-179. 10.1049/iet-syb.2008.0103
Dikicioglu D, Pir P, Onsan ZI, Ulgen KO, Kirdar B, Oliver SG: Integration of metabolic modeling and phenotypic data in evaluation and improvement of ethanol production using respiration-deficient mutants of Saccharomyces cerevisiae. Appl Environ Microbiol. 2008, 74: 5809-5816. 10.1128/AEM.00009-08
Bisson LF: The Biotechnology of Wine Yeast. Food Biotechnol. 2004, 18: 63-96. 10.1081/FBT-120030385.
Pretorius IS: Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast. 2000, 16: 675-729. 10.1002/1097-0061(20000615)16:8<675::AID-YEA585>3.0.CO;2-B
Pizarro F, Vargas FA, Agosin E: A systems biology perspective of wine fermentations. Yeast. 2007, 24: 977-991. 10.1002/yea.1545
Pharkya P, Maranas CD: An optimization framework for identifying reaction activation/inhibition or elimination candidates for overproduction in microbial systems. Metab Eng. 2006, 8: 1-13. 10.1016/j.ymben.2005.08.003
Patil KR, Rocha I, Förster J, Nielsen J: Evolutionary programming as a platform for in silico metabolic engineering. BMC Bioinformatics. 2005, 6: 308-320. 10.1186/1471-2105-6-308
Otero JM, Nielsen J: Industrial systems biology. Biotechnol Bioeng. 2009, 105: 439-460.
Mo ML, Palsson B, Herrgård MJ: Connecting extracellular metabolomic measurements to intracellular flux states in yeast. BMC Sys Biol. 2009, 3: 37-54. 10.1186/1752-0509-3-37.
Nookaew I, Jewett MC, Meechai A, Thammarongtham C, Laoteng K, Cheevadhanarak S, Nielsen J, Bhumiratana S: The genome-scale metabolic model iIN 800 of Saccharomyces cerevisiae and its validation: a scaffold to query lipid metabolism. BMC Sys Biol. 2008, 2: 71-10.1186/1752-0509-2-71.
Kuepfer L, Sauer U, Blank LM: Metabolic functions of duplicate genes in Saccharomyces cerevisiae. Genome Res. 2005, 15: 1421-1430. 10.1101/gr.3992505
Dobson PD, Smallbone K, Jameson D, Simeonidis E, Lanthaler K, Pir P, Lu C, Swainston N, Dunn WB, Fisher P: Further developments towards a genome-scale metabolic model of yeast. BMC Systems Biology. 2010, 4: 145-151. 10.1186/1752-0509-4-145
Varma A, Palsson BO: Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol. 1994, 60: 3724-3731.
Mahadevan R, Edwards JS, Doyle Iii FJ: Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys J. 2002, 83: 1331-1340. 10.1016/S0006-3495(02)73903-9
Anesiadis N, Cluett WR, Mahadevan R: Dynamic metabolic engineering for increasing bioprocess productivity. Metab Eng. 2008, 10: 255-266. 10.1016/j.ymben.2008.06.004
Gaspard L, Joeri B, Jo M, Ellen VH, Wim S, Erick V, Peter A: Dynamic Metabolic Flux Analysis Demonstrated on Cultures Where the Limiting Substrate Is Changed from Carbon to Nitrogen and Vice Versa. J Biomed Biotechnol. 2010, 2010: 19-
Lee JM, Gianchandani EP, Eddy JA, Papin JA: Dynamic analysis of integrated signaling, metabolic, and regulatory networks. PLoS Comput Biol. 2008, 4: e1000086- 10.1371/journal.pcbi.1000086
Sainz J, Pizarro F, Perez-Correa J, Agosin E: Modeling of yeast metabolism and process dynamics in batch fermentation. Biotechnol Bioeng. 2003, 81: 818-828. 10.1002/bit.10535
Luo R, Wei H, Ye L, Wang K, Chen F, Luo L, Liu L, Li Y, Crabbe MJC, Jin L: Photosynthetic metabolism of C3 plants shows highly cooperative regulation under changing environments: A systems biological analysis. Proc Natl Acad Sci USA. 2009, 106: 847-852. 10.1073/pnas.0810731105
Uygun K, Matthew HWT, Huang Y: DFBA-LQR: An optimal control approach to flux balance analysis. Ind Eng Chem Res. 2006, 45: 8554-8564. 10.1021/ie060218f.
Luo RY, Liao S, Tao GY, Li YY, Zeng S, Li YX, Luo Q: Dynamic analysis of optimality in myocardial energy metabolism under normal and ischemic conditions. Mol Syst Biol. 2006, 2: 1-6.
Salimi F, Zhuang K, Mahadevan R: Genome scale metabolic modeling of a clostridial co culture for consolidated bioprocessing. Biotechnologu. 2010, 5: 726-738.
Zhuang K, Izallalen M, Mouser P, Richter H, Risso C, Mahadevan R, Lovley DR: Genome- scale dynamic modeling of the competition between Rhodoferax and Geobacter in anoxic subsurface environments. The ISME Journal.
Meadows AL, Karnik R, Lam H, Forestell S, Snedecor B: Application of dynamic flux balance analysis to an industrial Escherichia coli fermentation. Metab Eng. 2009, 12: 150-160.
Oddone GM, Mills DA, Block DE: A dynamic, genome-scale flux model of Lactococcus lactis to increase specific recombinant protein expression. Metab Eng. 2009, 11: 367-381. 10.1016/j.ymben.2009.07.007
Famili I, Forster J, Nielsen J, Palsson BO: Saccharomyces cerevisiae Phenotypes Can Be Predicted by Using Constraint-Based Analysis of a Genome-Scale Reconstructed Metabolic Network. Proc Natl Acad Sci USA. 2003, 100: 13134-13139. 10.1073/pnas.2235812100
Edwards JS, Ibarra RU, Palsson BO: In silico predictions of Escherichia coli metabolic capabilities are consistent with experimental data. Nat Biotechnol. 2001, 19: 125-130. 10.1038/84379
Mahadevan R, Schilling CH: The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab Eng. 2003, 5: 264-276. 10.1016/j.ymben.2003.09.002
ter Linde JJM, Liang H, Davis RW, Steensma HY, van Dijken JP, Pronk JT: Genome-Wide Transcriptional Analysis of Aerobic and Anaerobic Chemostat Cultures of Saccharomyces cerevisiae. J Bacteriol. 1999, 181: 7409-7413.
van den Brink J, Daran-Lapujade P, Pronk JT, de Winde JH: New insights into the Saccharomyces cerevisiae fermentation switch: Dynamic transcriptional response to anaerobicity and glucose-excess. BMC Genomics. 2008, 9: 100- 10.1186/1471-2164-9-100
Boer VM, de Winde JH, Pronk JT, Piper MD: The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem. 2003, 278: 3265-3274. 10.1074/jbc.M209759200
Tai SL, Boer VM, Daran-Lapujade P, Walsh MC, de Winde JH, Daran JM, Pronk JT: Two- dimensional transcriptome analysis in chemostat cultures. Combinatorial effects of oxygen availability and macronutrient limitation in Saccharomyces cerevisiae. J Biol Chem. 2005, 280: 437-447.
Kwast KE, Lai LC, Menda N, James DT, Aref S, Burke PV: Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J Bacteriol. 2002, 184: 250-265. 10.1128/JB.184.1.250-265.2002
Verduyn C, Postma E, Scheffers WA, Van Dijken JP: Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Appl Microbiol. 1990, 136: 395-403.
Verduyn C, Stouthamer AH, Scheffers WA, Dijken JP: A theoretical evaluation of growth yields of yeasts. Antonie Van Leeuwenhoek. 1991, 59: 49-63. 10.1007/BF00582119
Bakker BM, Overkamp KM, van Maris AJ, Kotter P, Luttik MA, van Dijken JP, Pronk JT: Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2001, 25: 15-37. 10.1111/j.1574-6976.2001.tb00570.x
Lupiañez JA, Machado A, Nuñez de Castro I, Mayor F: Succinic acid production by yeasts grown under different hypoxic conditions. Mol Cell Biochem. 1974, 3: 113-116. 10.1007/BF01659183
Nissen TL, Schulze U, Nielsen J, Villadsen J: Flux distributions in anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae. Microbiology. 1997, 143: 203-218. 10.1099/00221287-143-1-203
Camarasa C, Grivet JP, Dequin S: Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology. 2003, 149: 2669-2678. 10.1099/mic.0.26007-0
Fornairon-Bonnefond C, Demaretz V, Rosenfeld E, Salmon JM: Oxygen addition and sterol synthesis in Saccharomyces cerevisiae during enological fermentation. J Biosci Bioeng. 2002, 93: 176-182. 10.1263/jbb.93.176
Andreasen AA, Stier TJB: Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cel Comp Physiol. 1953, 41: 23-26. 10.1002/jcp.1030410103.
Andreasen AA, Stier TJB: Anaerobic nutrition of saccharomyces cerevisiae. II. Unsaturated fatty and requirement for growth in a defined medium. J Cel Comp Physiol. 1954, 43: 271-281. 10.1002/jcp.1030430303.
Bisson LF: Stuck and sluggish fermentations. Am J Enol Viticul. 1999, 50: 107-
Perez M, Luyten K, Michel R, Riou C, Blondin B: Analysis of Saccharomyces cerevisiae hexose carrier expression during wine fermentation: both low- and high-affinity Hxt transporters are expressed. FEMS Yeast Res. 2005, 5: 351-361. 10.1016/j.femsyr.2004.09.005
Reifenberger E, Boles E, Ciriacy M: Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997, 245: 324-333. 10.1111/j.1432-1033.1997.00324.x
Luyten K, Riou C, Blondin B: The hexose transporters of Saccharomyces cerevisiae play different roles during enological fermentation. Yeast. 2002, 19: 713-726. 10.1002/yea.869
Gasch AP, Werner-Washburne M: The genomics of yeast responses to environmental stress and starvation. Funct Integr Genomics. 2002, 2: 181-192. 10.1007/s10142-002-0058-2
Rossouw D, Bauer FF: Comparing the transcriptomes of wine yeast strains: toward understanding the interaction between environment and transcriptome during fermentation. Appl Microbiol Biotechnol. 2009, 84: 937-954. 10.1007/s00253-009-2204-4
Piper PW, Talreja K, Panaretou B, Moradas-Ferreira P, Byrne K, Praekelt UM, Meacock P, Recnacq M, Boucherie H: Induction of major heat-shock proteins of Saccharomyces cerevisiae, including plasma membrane Hsp30, by ethanol levels above a critical threshold. Microbiology. 1994, 140: 3031-3038. 10.1099/13500872-140-11-3031
Pizarro FJ, Jewett MC, Nielsen J, Agosin E: Growth temperature exerts differential physiological and transcriptional responses in laboratory and wine strains of Saccharomyces cerevisiae. Appl Environ Microbiol. 2008, 74: 6358-6368. 10.1128/AEM.00602-08
Postmus J, Canelas AB, Bouwman J, Bakker BM, van Gulik W, de Mattos M, Brul S, Smits GJ: Quantitative analysis of the high temperature-induced glycolytic flux increase in Saccharomyces cerevisiae reveals dominant metabolic regulation. J Biol Chem. 2008, 283: 23524-23532. 10.1074/jbc.M802908200
Varela C, Pizarro F, Agosin E: Biomass content governs fermentation rate in nitrogen- deficient wine musts. Appl Environ Microbiol. 2004, 70: 3392-3400. 10.1128/AEM.70.6.3392-3400.2004
Pramanik J, Keasling JD: Effect of Escherichia coli biomass composition on central metabolic fluxes predicted by a stoichiometric model. Biotechnol Bioeng. 2000, 60: 230-238.
Cambon B, Monteil V, Remize F, Camarasa C, Dequin S: Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl Environ Microbiol. 2006, 72: 4688-4694. 10.1128/AEM.02975-05
Eglinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke PA, de Barros Lopes M: Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD 6 aldehyde dehydrogenase gene. Yeast. 2002, 19: 295-301. 10.1002/yea.834
Remize F, Roustan J, Sablayrolles J, Barre P, Dequin S: Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol. 1999, 65: 143-149.
Guo Z, Zhang L, Ding Z, Wang ZX, Shi GY: Interruption of glycerol pathway in industrial alcoholic yeasts to improve the ethanol production. Appl Microbiol Biotechnol. 2009, 82: 287-292. 10.1007/s00253-008-1777-7
Remize F, Andrieu E, Dequin S: Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: role of the cytosolic Mg2+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl Environ Microbiol. 2000, 66: 3151-3159. 10.1128/AEM.66.8.3151-3159.2000
Saint-Prix F, Bonquist L, Dequin S: Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: the NADP+-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology. 2004, 150: 2209-2220. 10.1099/mic.0.26999-0
Zhang A, Kong Q, Cao L, Chen X: Effect of FPS1 deletion on the fermentation properties of Saccharomyces cerevisiae. Lett Appl Microbiol. 2007, 44: 212-217. 10.1111/j.1472-765X.2006.02041.x
Guadalupe Medina V, Almering MJH, van Maris AJA, Pronk JT: Elimination of glycerol production in anaerobic cultures of a Saccharomyces cerevisiae strain engineered to use acetic acid as an electron acceptor. Appl Environ Microbiol. 2010, 76: 190-195. 10.1128/AEM.01772-09
Herrgård MJ, Lee BS, Portnoy V, Palsson B: Integrated analysis of regulatory and metabolic networks reveals novel regulatory mechanisms in Saccharomyces cerevisiae. Genome Res. 2006, 16: 627-635. 10.1101/gr.4083206
Covert MW, Palsson BO: Constraints-based models: regulation of gene expression reduces the steady-state solution space. J Theor Biol. 2003, 221: 309-325. 10.1006/jtbi.2003.3071
Acknowledgements
The authors thank Drs. Sylvie Dequin and Carole Camarasa for providing fermentation data to validate the model. The authors also thank Dr. Jens Nielsen for providing the genome-scale metabolic network. This work was funded by VINNOVA 05CTE01-06. FAV acknowledges support by CONICYT. We also acknowledge the suggestions of the anonymous referees that contributed to significantly improve our manuscript. Finally, we are grateful to Lisa Gingles for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
The authors FAV, FP, JRP and EA, conceived and designed the experiments. FAV performed the experiments and analyzed the data. All authors have read and approved the final manuscript.
Electronic supplementary material
12918_2010_666_MOESM1_ESM.PDF
Additional file 1: Sensitivity analyses of the idFV715 model. This file includes sensitivity analyses of the effect of time step integration and acetate production in the idFV715 model. (PDF 332 KB)
12918_2010_666_MOESM2_ESM.PDF
Additional file 2: Kinetic expression used in the idFV715 model. This file includes kinetic expression used in the idFV715 model. Sugar, nitrogen and maintenance expressions are detailed. (PDF 308 KB)
12918_2010_666_MOESM3_ESM.PDF
Additional file 3: Model validation. This file includes tables where results using previous DFBA model can be directly compared to current model results of Table 4. Also, a description of metabolic engineering conditions was included. (PDF 242 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vargas, F.A., Pizarro, F., Pérez-Correa, J.R. et al. Expanding a dynamic flux balance model of yeast fermentation to genome-scale. BMC Syst Biol 5, 75 (2011). https://doi.org/10.1186/1752-0509-5-75
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-0509-5-75