Abstract
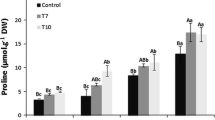
Vegetable soybeans [Glycine max (L.) Merrill] are susceptible to salt stress and, thus, soil salinity can severely affect their growth and productivity. To enhance the salt tolerance of vegetable soybeans, a novel Solanum torvum Swartz Δ1-pyrroline-5-carboxylate synthetase gene (StP5CS, GenBank accession number: JN606861) that encodes a critical regulatory enzyme in proline biosynthesis was transformed into the cultivar NY-1001 using an Agrobacterium-mediated transformation method. PCR and Southern blot analyses indicated that two independent T0 fertile transgenic plants were generated. The transgenic plants transmitted the transgenes into their T1 progeny in a 3:1 ratio. The T2 and T3 homozygous transgenic lines (HTLs) were examined for salt tolerance in pot and hydroponic cultures, respectively. The StP5CS overexpression conferred salt tolerance in T2 and T3 HTLs. Under NaCl stress conditions, the leaf scorch scores of T2 and T3 HTLs were significantly lower than those of wild-type (WT) plants. The plant height, leaf area, relative chlorophyll content, and number of fresh pods of T2 and T3 HTLs were significantly higher than those of WT plants. Compared with WT plants, T2 and T3 HTLs had significantly higher levels of proline and significantly lower levels of membrane lipid peroxidation. These results indicate that StP5CS overexpression in HTLs results in enhanced salt tolerance associated with higher levels of proline accumulation under salinity stress and that StP5CS can be utilized to improve salinity tolerance in vegetable crop genetic engineering.
Similar content being viewed by others
Literature Cited
Ashraf, M. and N.A. Akram. 2009. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 27:744–752.
Bates, L.S., R.P. Waldren, and I.D. Teare. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207.
Cao, W.H., J. Liu, X.J. He, R.L. Mu, H.L. Zhou, S.Y. Chen, and J.S. Zhang. 2007. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 143:707–719.
Çelik, Ö. and S.G. Ünsal. 2013. Expression analysis of proline metabolism-related genes in salt-tolerant soybean mutant plants. Plant Omics 6:364–370.
Chakraborty, K., R.K. Sairam, and R.C. Bhattacharya. 2012. Salinity-induced expression of pyrrolline-5-carboxylate synthetase determine salinity tolerance in Brassica spp. Acta Physiol. Plant. 34:1935–1941.
Chen, G.H., W. Yan, L.F. Yang, J.Y. Gai, and Y.L. Zhu. 2014. Overexpression of StNHX1, a novel vacuolar Na+/H+ antiporter gene from Solanum torvum, enhances salt tolerance in transgenic vegetable soybean. Hort. Environ. Biotechnol. 55:213–221.
Chen, P., K. Yan, H. Shao, and S. Zhao. 2013a. Physiological mechanisms for high salt tolerance in wild soybean (Glycine soja) from Yellow River Delta, China: Photosynthesis, osmotic regulation, ion flux and antioxidant capacity. PloS One 8:e83227.
Chen, J.B., J.W. Yang, Z.Y. Zhang, X.F. Feng, and S.M. Wang. 2013b. Two P5CS genes from common bean exhibiting different tolerance to salt stress in transgenic Arabidopsis. J. Genet. 92:461–469.
Choi, H.J., T. Chandrasekhar, H.Y. Lee, and K.M. Kim. 2007. Production of herbicide-resistant transgenic sweet potato plants through Agrobacterium tumefaciens method. Plant Cell Tiss. Organ Cult. 91:235–242.
De Carvalho, K., M.K.F. De Campos, D.S. Domingues, L.F.P. Pereira, and L.G.E. Vieira. 2013. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 40:3269–3279.
Hou, J., C. Wang, X. Hong, J. Zhao, C. Xue, N. Guo, J. Gai, and H. Xing. 2011. Association analysis of vegetable soybean quality traits with SSR markers. Plant Breed. 130:444–449.
Jackson, M.A., D.J. Anderson, and R.G. Birch. 2013. Comparison of Agrobacterium and particle bombardment using whole plasmid or minimal cassette for production of high-expressing, low-copy transgenic plants. Transgenic Res. 22:143–151.
Jefferson, R.A., T.A. Kavanagh, and M.W. Bevan. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901–3907.
Jyothishwaran, G., D. Kotresha, T. Selvaraj, S. Srideshikan, P. Rajvanshi, and C. Jayabaskaran. 2007. A modified freeze-thaw method for efficient transformation of Agrobacterium tumefaciens. Curr. Sci. 93:770–772.
Karthikeyan, A., S.K. Pandian, and M. Ramesh. 2011. Transgenic indica rice cv. ADT 43 expressing a Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. Plant Cell Tiss. Organ Cult. 107:383–395.
Kavi Kishor, P.B., Z. Hong, G.H. Miao, C.-A.A. Hu, and D.P.S. Verma. 1995. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 8:1387–1394.
Kim, G.B. and Y.W. Nam. 2013. A novel Δ1-pyrroline-5-carboxylate synthetase gene of Medicago truncatula plays a predominant role in stress-induced proline accumulation during symbiotic nitrogen fixation. J. Plant Physiol. 170:291–302.
Le, D.T., D.L. Aldrich, B. Valliyodan, Y. Watanabe, C. Van Ha, R. Nishiyama, S.K. Guttikonda, T.N. Quach, J.J. Gutierrez-Gonzalez, T. Lam-Son Phan, and H.T. Nguyen. 2012. Evaluation of candidate reference genes for normalization of quantitative RT-PCR in soybean tissues under various abiotic stress conditions. PloS One 7:e46487.
Lee, J.D., S.L. Smothers, D. Dunn, M. Villagarcia, C.R. Shumway, T.E. Carter, Jr., and J.G. Shannon. 2008. Evaluation of a simple method to screen soybean genotypes for salt tolerance. Crop Sci. 48:2194–2200.
Liu, S.C., G.H. Chen, L.F. Yang, J.Y. Gai, and Y.L. Zhu. 2013. Production of transgenic soybean to eliminate the major allergen Gly m Bd 30K by RNA interference-mediated gene silencing. J. Pure Appl. Microbiol. 7(Suppl.):589–599.
Liu, S.C., G.C. Zhang, L.F. Yang, M. Mii, J.Y. Gai, and Y.L. Zhu. 2014. Bialaphos-resistant transgenic soybeans produced by the Agrobacterium-mediated cotyledonary-node method. J. Agric. Sci. Technol. 16:175–190.
Mariashibu, T.S., K. Subramanyam, M. Arun, S. Mayavan, M. Rajesh, J. Theboral, M. Manickavasagam, and A. Ganapathi. 2013. Vacuum infiltration enhances the Agrobacterium-mediated genetic transformation in Indian soybean cultivars. Acta Physiol. Plant. 35:41–54.
Mattioli, R., P. Costantino, and M. Trovato. 2009. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 4:1016–1018.
Murray, M.G. and W.F. Thompson. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 9:4321–4325.
Phang, T.H., G.H. Shao, and H.M. Lam. 2008. Salt tolerance in soybean. J. Integr. Plant Biol. 50:1196–1212.
Rengasamy, P. 2010. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 37:613–620.
Richardson, K.A., D.A. Maher, C.S. Jones, and G. Bryan. 2013. Genetic transformation of western clover (Trifolium occidentale DE Coombe.) as a model for functional genomics and transgene introgression in clonal pasture legume species. Plant Methods 9:25.
Rivero, R.M., T.C. Mestre, R. Mittler, F. Rubio, F. Garcia-Sanchez, and V. Martinez. 2014. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 37:1059–1073.
Rodal, A.A., A.L. Manning, B.L. Goode, and D.G. Drubin. 2003. Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr. Biol. 13:1000–1008.
Ruiz-Lozano, J.M., R. Porcel, C. Azcon, and R. Aroca. 2012. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J. Exp. Bot. 63:4033–4044.
Schmittgen, T.D. and K.J. Livak. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3:1101–1108.
Shi, H., B.H. Lee, S.J. Wu, and J.K. Zhu. 2002. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat. Biotechnol. 21:81–85.
Song, J., C. Liu, D. Li, and Z. Gu. 2013. Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max L. Merr.). Ind. Crop. Prod. 50:743–749.
Surekha, C., K. Nirmala Kumari, L. Aruna, G. Suneetha, A. Arundhati, and P.B. Kavi Kishor. 2014. Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tiss. Organ Cult. 116:27–36.
Székely, G., E. Ábrahám, Á. Cséplő, G. Rigó, L. Zsigmond, J. Csiszár, F. Ayaydin, N. Strizhov, J. Jásik, and E. Schmelzer. 2008. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 53:11–28.
Szabados, L. and A. Savouré. 2010. Proline: a multifunctional amino acid. Trends Plant Sci. 15:89–97.
Vendruscolo, E.C.G., I. Schuster, M. Pileggi, C.A. Scapim, H.B.C. Molinari, C.J. Marur, and L.G.E. Vieira. 2007. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 164:1367–1376.
Verbruggen, N. and C. Hermans. 2008. Proline accumulation in plants: A review. Amino Acids 35:753–759.
Wei, G.P., L.F. Yang, Y.L. Zhu, and G. Chen. 2009. Changes in oxidative damage, antioxidant enzyme activities and polyamine contents in leaves of grafted and non-grafted eggplant seedlings under stress by excess of calcium nitrate. Sci. Hort. 120:443–451.
Zhang, G.W., S.C. Xu, W.H. Mao, Q.Z. Hu, and Y.M. Gong. 2013a. Determination of the genetic diversity of vegetable soybean Glycine max (L.) Merr. using EST-SSR markers. J. Zhejiang Univ.-SCI. B 14:279–288.
Zhang, X.X., Y.J. Tang, Q.B. Ma, C.Y. Yang, Y.H. Mu, H.C. Suo, L.H. Luo, and H. Nian. 2013b. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PloS One 8:e83011.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, GC., Zhu, WL., Gai, JY. et al. Enhanced salt tolerance of transgenic vegetable soybeans resulting from overexpression of a novel Δ1-pyrroline-5-carboxylate synthetase gene from Solanum torvum Swartz. Hortic. Environ. Biotechnol. 56, 94–104 (2015). https://doi.org/10.1007/s13580-015-0084-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-015-0084-3