Abstract
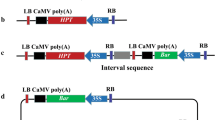
Transgene integration complexity in the recipient genome can be an important determinant of transgene expression and field performance in transgenic crops. We provide the first direct comparison of Agrobacterium-mediated transformation (AMT) and particle bombardment using whole plasmid (WP) and excised minimal cassettes (MC), for transformation efficiency, transgene integration complexity and transgene expression in plants. To enable direct comparison, a selectable marker and a luciferase reporter gene were linked in identical configurations in plasmids suitable for AMT or direct gene transfer into sugarcane. Transformation efficiencies were similar between WP and MC when equal molar DNA quantities were delivered. When the MC concentration was reduced from 66 to 6.6 ng per shot, transformation efficiency dropped fourfold, to a level equivalent with AMT in amenable genotype Q117. The highest proportion of transformants combining low copy number (estimated below two integrated copies by qPCR) with expression of the non-selected reporter gene was obtained using AMT (55 %) or MC at low DNA concentration (30 %). In sugarcane, both of these methods yielded high-expressing, single-copy transgenic plant lines at a workable efficiency for practical plant improvement; but AMT is currently limited to a few amenable genotypes. These methods are best coupled with rapid early screens for desired molecular characteristics of transformants, e.g. PCR screens for low copy number and/or transcription of the gene of practical interest.




Similar content being viewed by others
Abbreviations
- AMT:
-
Agrobacterium-mediated transformation
- CNI:
-
Copy number index
- LUC:
-
Luciferase
- MC:
-
Minimal cassette
- WP:
-
Whole plasmid
References
Agrawal PK, Kohli A, Twyman RM, Christou P (2005) Transformation of plants with multiple cassettes generates simple transgene integration patterns and high expression levels. Mol Breed 16:247–260
Akbudak MA, More AB, Nandy S, Srivastava V (2010) Dosage-dependent gene expression from direct repeat locus in rice developed by site-specific gene integration. Mol Biotechnol 45:15–23. doi:10.1007/s12033-009-9235-z
Altpeter F, Baisakh N, Beachy R, Bock R, Capell T, Christou P, Daniell H, Datta K, Datta S, Dix PJ, Fauquet C, Huang N, Kohli A, Mooibroek H, Nicholson L, Nguyen TT, Nugent G, Raemakers K, Romano A, Somers DA, Stoger E, Taylor N, Visser R (2005) Particle bombardment and the genetic enhancement of crops: myths and realities. Mol Breed 15:305–327
Anderson DJ, Birch RG (2012) Minimal handling and super-binary vectors facilitate efficient, Agrobacterium-mediated, transformation of sugarcane (Saccharum spp. hybrid). Trop Plant Biol. doi: 10.1007/s12042-012-9101-1
Basnayake SWV, Moyle R, Birch RG (2011) Embryogenic callus proliferation and regeneration conditions for genetic transformation of diverse sugarcane cultivars. Plant Cell Rep 30:439–448. doi:10.1007/s00299-010-0927-4
Birch RG, Bower RS, Elliott AR (2010) Highly efficient, 5′-sequence-specific transgene silencing in a complex polyploid. Trop Plant Biol 3:88–97
Bower R, Birch RG (1992) Transgenic sugarcane plants via microprojectile bombardment. Plant J 2:409–416
Bower R, Elliott AR, Potier BAM, Birch RG (1996) High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol Breed 2:239–249
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Breitler JC, Labeyrie A, Meynard D, Legavre T, Guiderdoni E (2002) Efficient microprojectile bombardment-mediated transformation of rice using gene cassettes. Theor Appl Genet 104:709–719. doi:10.1007/s00122-001-0786-z
Chawla R, Ariza-Nieto M, Wilson AJ, Moore SK, Srivastava V (2006) Transgene expression produced by biolistic-mediated, site-specific gene integration is consistently inherited by the subsequent generations. Plant Biotechnol J 4:209–218. doi:10.1111/j.1467-7652.2005.00173.x
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes - structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Christou P (1995) Strategies for variety-independent genetic transformation of important cereals, legumes and woody species utilizing particle bombardment. Euphytica 85:13–27
De Buck S, De Wilde C, Van Montagu M, Depicker A (2000) T-DNA vector backbone sequences are frequently integrated into the genome of transgenic plants obtained by Agrobacterium-mediated transformation. Mol Breed 6:459–468
Fu XD, Duc LT, Fontana S, Bong BB, Tinjuangjun P, Sudhakar D, Twyman RM, Christou P, Kohli A (2000) Linear transgene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res 9:11–19
Graham MW, Mudge SR, Sternes PR, Birch RG (2011) Understanding and avoiding transgene silencing. In: Stewart CN, Touraev A, Citovsky V, Tzfira T (eds) Plant transformation technologies. Wiley-Blackwell, Chichester, pp 171–196
Iskandar HM, Casu RE, Fletcher AT, Schmidt S, Xu JS, Maclean DJ, Manners JM, Bonnett GD (2011) Identification of drought-response genes and a study of their expression during sucrose accumulation and water deficit in sugarcane culms. BMC Plant Biol 11:12. doi:10.1186/1471-2229-11-12
Jayaraj J, Liang GH, Muthukrishnan S, Punja ZK (2008) Generation of low copy number and stably expressing transgenic creeping bentgrass plants using minimal gene cassette bombardment. Biol Plant 52:215–221
Jordan MC (2000) Green fluorescent protein as a visual marker for wheat transformation. Plant Cell Rep 19:1069–1075
Kim SR, Lee J, Jun SH, Park S, Kang HG, Kwon S, An G (2003) Transgene structures in T-DNA-inserted rice plants. Plant Mol Biol 52:761–773
Kim JY, Gallo M, Altpeter F (2012) Analysis of transgene integration and expression following biolistic transfer of different quantities of minimal expression cassette into sugarcane (Saccharum spp. hybrids). Plant Cell Tiss Org Cult 108:297–302. doi:10.1007/s11240-011-0043-3
Kohli A, Twyman RM, Abranches R, Wegel E, Stoger E, Christou P (2003) Transgene integration, organization and interaction in plants. Plant Mol Biol 52:247–258
Koncz C, Degreve H, Andre D, Deboeck F, Vanmontagu M, Schell J (1983) The opine synthase genes carried by Ti plasmids contain all signals necessary for expression in plants. EMBO J 2:1597–1603
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Bio/Technol 9:963–967
Loc NT, Tinjuangjun P, Gatehouse AMR, Christou P, Gatehouse JA (2002) Linear transgene constructs lacking vector backbone sequences generate transgenic rice plants which accumulate higher levels of proteins conferring insect resistance. Mol Breed 9:231–244
Lowe BA, Prakash NS, Way M, Mann MT, Spencer TM, Boddupalli RS (2009) Enhanced single copy integration events in corn via particle bombardment using low quantities of DNA. Transgenic Res 18:831–840. doi:10.1007/s11248-009-9265-0
Luehrsen KR, Walbot V (1993) Firefly luciferase as a reporter for plant gene expression studies. Promega Notes 44:24–29
Luo ZH, Chen ZX (2007) Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell 19:943–958
Meng L, Ziv M, Lemaux PG (2006) Nature of stress and transgene locus influences transgene expression stability in barley. Plant Mol Biol 62:15–28. doi:10.1007/s11103-006-9000-7
Olhoft PM, Flagel LE, Somers DA (2004) T-DNA locus structure in a large population of soybean plants transformed using the Agrobacterium-mediated cotyledonary-node method. Plant Biotechnol J 2:289–300. doi:10.1111/j.1467-7652.2004.00070.x
Oltmanns H, Frame B, Lee LY, Johnson S, Li B, Wang K, Gelvin SB (2010) Generation of backbone-free, low transgene copy plants by launching T- DNA from the Agrobacterium chromosome. Plant Physiol 152:1158–1166. doi:10.1104/pp.109.148585
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi:10.1093/nar/29.9.e45
Prakash NS, Bhojaraja R, Shivbachan SK, Priya GGH, Nagraj TK, Prasad V, Babu VS, Jayaprakash TL, Dasgupta S, Spencer TM, Boddupalli RS (2009) Marker-free transgenic corn plant production through co-bombardment. Plant Cell Rep 28:1655–1668. doi:10.1007/s00299-009-0765-4
Rogers S, Bendich A (1988) Extraction of DNA from plant tissues. In: Gelvin SB, Schilperoort RA, Verma DPS (eds) Plant molecular biology manual. Kluwer Academic Publishers, Dordrecht, pp 1–10
Romano A, Raemakers K, Bernardi J, Visser R, Mooibroek H (2003) Transgene organisation in potato after particle bombardment-mediated (co-)transformation using plasmids and gene cassettes. Transgenic Res 12:461–473. doi:10.1023/a:1024267906219
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sandhu S, Altpeter F (2008) Co-integration, co-expression and inheritance of unlinked minimal transgene expression cassettes in an apomictic turf and forage grass (Paspalum notatum Flugge). Plant Cell Rep 27:1755–1765. doi:10.1007/s00299-008-0599-5
Schubert D, Lechtenberg B, Forsbach A, Gils M, Bahadur S, Schmidt R (2004) Silencing in Arabidopsis T-DNA transformants: the predominant role of a gene-specific RNA sensing mechanism versus position effects. Plant Cell 16:2561–2572. doi:10.1105/tpc.104.024547
Shrawat AK, Lorz H (2006) Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol J 4:575–603
Srivastava V, Gidoni D (2010) Site-specific gene integration technologies for crop improvement. In Vitro Cell Dev Biol Plant 46:219–232. doi:10.1007/s11627-009-9274-y
Thomas-Hall S, Campbe PR, Carlens K, Kawanishi E, Swennen R, Sagi L, Schenk PM (2007) Phylogenetic and molecular analysis of the ribulose-1,5-bisphosphate carboxylase small subunit gene family in banana. J Exp Bot 58:2685–2697. doi:10.1093/Jxb/Erm129
Wu HX, Doherty A, Jones HD (2008) Efficient and rapid Agrobacterium-mediated genetic transformation of durum wheat (Triticum turgidum L-var. durum) using additional virulence genes. Transgenic Res 17:425–436
Yao Q, Cong L, Chang JL, Li KX, Yang GX, He GY (2006) Low copy number gene transfer and stable expression in a commercial wheat cultivar via particle bombardment. J Exp Bot 57:3737–3746. doi:10.1093/jxb/erl145
Acknowledgments
This work was undertaken through a collaboration between CSR Sugar Limited (Sucrogen) and The University of Queensland with support from the Australian Research Council. The authors acknowledge the excellent technical assistance of Andrea Gray, Kuang-Yu Chen, Mona Singh and Kerrin Henderson.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mark A. Jackson, David J. Anderson contributed equally toward this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2012_9639_MOESM1_ESM.eps
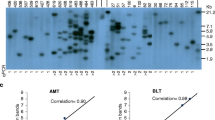
Fig. S1 LUC activity profiles across populations of transgenic plants produced using methods explained in Table 1. LUC activity was measured in extracts from young leaf tissue (EPS 263 kb)
Rights and permissions
About this article
Cite this article
Jackson, M.A., Anderson, D.J. & Birch, R.G. Comparison of Agrobacterium and particle bombardment using whole plasmid or minimal cassette for production of high-expressing, low-copy transgenic plants. Transgenic Res 22, 143–151 (2013). https://doi.org/10.1007/s11248-012-9639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-012-9639-6