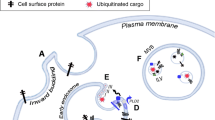
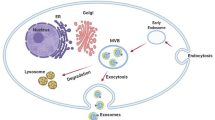
Abstract
Background
Recent advances in cancer biology have highlighted the relevance of exosomes and nanovesicles as carriers of genetic and biological messages between cancer cells and their immediate and/or distant environments. It has been found that these molecular cues may play significant roles in cancer progression and metastasis. Cancer cells secrete exosomes containing diverse molecules that can be transferred to recipient cells and/or vice versa to induce a plethora of biological processes, including angiogenesis, metastasis formation, therapeutic resistance, epithelial-mesenchymal transition and epigenetic/stemness (re)programming. While exosomes interact with cells within the tumour microenvironment to promote tumour growth, these vesicles can also facilitate the process of distant metastasis by mediating the formation of pre-metastatic niches. Next to their tumour promoting effects, exosomes have been found to serve as potential tools for cancer diagnosis and therapy. The ease of isolating exosomes and their content from different body fluids has led to the identification of diagnostic and prognostic biomarker signatures, as well as to predictive biomarker signatures for therapeutic responses. Exosomes can also be used as cargos to deliver therapeutic anti-cancer drugs, and they can be engineered to serve as vaccines for immunotherapy. Additionally, it has been found that inhibition of exosome secretion, and thus the transfer of oncogenic molecules, holds promise for inhibiting tumour growth. Here we provide recent information on the diverse roles of exosomes in various cellular and systemic processes governing cancer progression, and discuss novel strategies to halt this progression using exosome-based targeted therapies and methods to inhibit exosome secretion and the transfer of pro-tumorigenic molecules.
Conclusions
This review highlights the important role of exosomes in cancer progression and its implications for (non-invasive) diagnostics and the development of novel therapeutic strategies, as well as its current and future applications in clinical trials.


Similar content being viewed by others
Change history
25 July 2018
In the title of above mentioned article the word ‘versatile’ had been replaced by ‘multifaceted’.
References
S.E.L. Andaloussi, I. Mager, X.O. Breakefield, M.J. Wood, Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013)
R. Kalluri, M. Zeisberg, Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006)
D.D. Yu, Y. Wu, H.Y. Shen, M.M. Lv, W.X. Chen, X.H. Zhang, S.L. Zhong, J.H. Tang, J.H. Zhao, Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 106, 959–964 (2015)
C. Thery, L. Zitvogel, S. Amigorena, Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002)
L. Muller, M. Mitsuhashi, P. Simms, W.E. Gooding, T.L. Whiteside, Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 6, 20254 (2016)
H. Zhao, L. Yang, J. Baddour, A. Achreja, V. Bernard, T. Moss, J.C. Marini, T. Tudawe, E.G. Seviour, F.A. San Lucas, H. Alvarez, S. Gupta, S.N. Maiti, L. Cooper, D. Peehl, P.T. Ram, A. Maitra, D. Nagrath, Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. elife 5, e10250 (2016)
A.S. Azmi, B. Bao, F.H. Sarkar, Exosomes in cancer development, metastasis and drug resistance: a comprehensive review. Cancer Metastasis Rev. 32, 623–642 (2013). https://doi.org/10.1007/s10555-013-9441-9
S.N. Chatterjee, J. Das, Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J. Gen. Microbiol. 49, 1–11 (1967)
T.N. Ellis, M.J. Kuehn, Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 (2010)
X. Yu, S.L. Harris, A.J. Levine, The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 66, 4795–4801 (2006)
A. Lespagnol, D. Duflaut, C. Beekman, L. Blanc, G. Fiucci, J.C. Marine, M. Vidal, R. Amson, A. Telerman, Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 15, 1723–1733 (2008)
C. Thery, M. Ostrowski, E. Segura, Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009)
W. Li, Y. Hu, T. Jiang, Y. Han, G. Han, J. Chen, X. Li, Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS 122, 1080–1087 (2014)
I. Parolini, C. Federici, C. Raggi, L. Lugini, S. Palleschi, A. De Milito, C. Coscia, E. Iessi, M. Logozzi, A. Molinari, M. Colone, M. Tatti, M. Sargiacomo, S. Fais, Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 (2009)
J. Faure, G. Lachenal, M. Court, J. Hirrlinger, C. Chatellard-Causse, B. Blot, J. Grange, G. Schoehn, Y. Goldberg, V. Boyer, F. Kirchhoff, G. Raposo, J. Garin, R. Sadoul, Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 (2006)
G. Lachenal, K. Pernet-Gallay, M. Chivet, F.J. Hemming, A. Belly, G. Bodon, B. Blot, G. Haase, Y. Goldberg, R. Sadoul, Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 46, 409–418 (2011)
N. Blanchard, D. Lankar, F. Faure, A. Regnault, C. Dumont, G. Raposo, C. Hivroz, TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 168, 3235–3241 (2002)
C.T. Roberts Jr., P. Kurre, Vesicle trafficking and RNA transfer add complexity and connectivity to cell-cell communication. Cancer Res. 73, 3200–3205 (2013)
E.G. Trams, C.J. Lauter, N. Salem Jr., U. Heine, Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645, 63–70 (1981)
B.T. Pan, R.M. Johnstone, Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978 (1983)
C. Harding, J. Heuser, P. Stahl, Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 35, 256–263 (1984)
L. Balaj, R. Lessard, L. Dai, Y.J. Cho, S.L. Pomeroy, X.O. Breakefield, J. Skog, Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011)
H. Valadi, K. Ekstrom, A. Bossios, M. Sjostrand, J.J. Lee, J.O. Lotvall, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007)
C. Admyre, S.M. Johansson, K.R. Qazi, J.J. Filen, R. Lahesmaa, M. Norman, E.P. Neve, A. Scheynius, S. Gabrielsson, Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978 (2007)
M.P. Caby, D. Lankar, C. Vincendeau-Scherrer, G. Raposo, C. Bonnerot, Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 17, 879–887 (2005)
R. Shi, P.Y. Wang, X.Y. Li, J.X. Chen, Y. Li, X.Z. Zhang, C.G. Zhang, T. Jiang, W.B. Li, W. Ding, S.J. Cheng, Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 6, 26971–26981 (2015)
M. Gonzalez-Begne, B. Lu, X. Han, F.K. Hagen, A.R. Hand, J.E. Melvin, J.R. Yates, Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J. Proteome Res. 8, 1304–1314 (2009)
M. Tokuhisa, Y. Ichikawa, N. Kosaka, T. Ochiya, M. Yashiro, K. Hirakawa, T. Kosaka, H. Makino, H. Akiyama, C. Kunisaki, I. Endo, Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One 10, e0130472 (2015)
T. Pisitkun, R.F. Shen, M.A. Knepper, Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U. S. A. 101, 13368–13373 (2004)
D.D. Taylor, C. Gercel-Taylor, MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 (2008)
C. Thery, S. Amigorena, G. Raposo and A. Clayton, Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3 22 (2006)
K. Trajkovic, C. Hsu, S. Chiantia, L. Rajendran, D. Wenzel, F. Wieland, P. Schwille, B. Brugger, M. Simons, Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008)
T. Wollert, J.H. Hurley, Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464, 864–869 (2010)
T. Ravid, J.M. Heidinger, P. Gee, E.M. Khan, T. Goldkorn, c-Cbl-mediated ubiquitinylation is required for epidermal growth factor receptor exit from the early endosomes. J. Biol. Chem. 279, 37153–37162 (2004)
L. Duan, Y. Miura, M. Dimri, B. Majumder, I.L. Dodge, A.L. Reddi, A. Ghosh, N. Fernandes, P. Zhou, K. Mullane-Robinson, N. Rao, S. Donoghue, R.A. Rogers, D. Bowtell, M. Naramura, H. Gu, V. Band, H. Band, Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J. Biol. Chem. 278, 28950–28960 (2003)
O. Schmidt, D. Teis, The ESCRT machinery. Curr. Biol. 22, R116–R120 (2012)
S. Stuffers, C. Sem Wegner, H. Stenmark, A. Brech, Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10, 925–937 (2009)
T. Kajimoto, T. Okada, S. Miya, L. Zhang, S. Nakamura, Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 4, 2712 (2013)
D. Perez-Hernandez, C. Gutierrez-Vazquez, I. Jorge, S. Lopez-Martin, A. Ursa, F. Sanchez-Madrid, J. Vazquez, M. Yanez-Mo, The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 288, 11649–11661 (2013)
A.V. Vlassov, S. Magdaleno, R. Setterquist, R. Conrad, Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 1820, 940–948 (2012)
M. Record, K. Carayon, M. Poirot, S. Silvente-Poirot, Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 1841, 108–120 (2014)
F. Coutant, L. Perrin-Cocon, S. Agaugué, T. Delair, P. André, V. Lotteau, Mature dendritic cell generation promoted by lysophosphatidylcholine. J. Immunol. 169, 1688–1695 (2002)
L. Perrin-Cocon, S. Agaugué, F. Coutant, A. Masurel, S. Bezzine, G. Lambeau, P. André, V. Lotteau, Secretory phospholipase A2 induces dendritic cell maturation. Eur. J. Immunol. 34, 2293–2302 (2004)
Q. Ge, Y. Zhou, J. Lu, Y. Bai, X. Xie, Z. Lu, miRNA in plasma exosome is stable under different storage conditions. Molecules 19, 1568–1575 (2014)
C. Kahlert, R. Kalluri, Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 91, 431–437 (2013)
L.A. Mulcahy, R.C. Pink and D.R.F. Carter, Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, https://doi.org/10.3402/jev.v3403.24641 (2014)
T. Tian, Y.L. Zhu, Y.Y. Zhou, G.F. Liang, Y.Y. Wang, F.H. Hu, Z.D. Xiao, Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 289, 22258–22267 (2014)
K.J. Svensson, H.C. Christianson, A. Wittrup, E. Bourseau-Guilmain, E. Lindqvist, L.M. Svensson, M. Morgelin, M. Belting, Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288, 17713–17724 (2013)
D. Zech, S. Rana, M.W. Büchler, M. Zöller, Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun. Signaling 10, 37 (2012)
S. Rana, S. Yue, D. Stadel, M. Zoller, Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 44, 1574–1584 (2012)
T.I. Naslund, D. Paquin-Proulx, P.T. Paredes, H. Vallhov, J.K. Sandberg, S. Gabrielsson, Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 28, 171–180 (2014)
J.R. Goldenring, A central role for vesicle trafficking in epithelial neoplasia: intracellular highways to carcinogenesis. Nat. Rev. Cancer 13, 813–820 (2013)
N. Jae, D.G. McEwan, Y. Manavski, R.A. Boon, S. Dimmeler, Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 589, 3182–3188 (2015)
S.N. Hurwitz, M.M. Conlon, M.A. Rider, N.C. Brownstein, D.G. Meckes Jr., Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles 5, 31295 (2016)
C. Hsu, Y. Morohashi, S. Yoshimura, N. Manrique-Hoyos, S. Jung, M.A. Lauterbach, M. Bakhti, M. Gronborg, W. Mobius, J. Rhee, F.A. Barr, M. Simons, Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232 (2010)
C.A. Thompson, A. Purushothaman, V.C. Ramani, I. Vlodavsky, R.D. Sanderson, Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 288, 10093–10099 (2013)
A. Savina, C.M. Fader, M.T. Damiani, M.I. Colombo, Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6, 131–143 (2005)
H.W. King, M.Z. Michael, J.M. Gleadle, Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12, 1–10 (2012)
A.Z. Ayob, T.S. Ramasamy, Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 25, 20 (2018)
K. Al-Nedawi, B. Meehan, J. Micallef, V. Lhotak, L. May, A. Guha, J. Rak, Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008)
M.M. Valenzuela, H.R. Ferguson Bennit, A. Gonda, C.J. Diaz Osterman, A. Hibma, S. Khan, N.R. Wall, Exosomes secreted from human cancer cell lines contain inhibitors of apoptosis (IAP). Cancer Microenviron. 8, 65–73 (2015)
E. Donnarumma, D. Fiore, M. Nappa, G. Roscigno, A. Adamo, M. Iaboni, V. Russo, A. Affinito, I. Puoti, C. Quintavalle, A. Rienzo, S. Piscuoglio, R. Thomas, G. Condorelli, Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 8, 19592–19608 (2017)
A. Ramteke, H. Ting, C. Agarwal, S. Mateen, R. Somasagara, A. Hussain, M. Graner, B. Frederick, R. Agarwal, G. Deep, Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 54, 554–565 (2015)
S. Bao, Q. Wu, S. Sathornsumetee, Y. Hao, Z. Li, A.B. Hjelmeland, Q. Shi, R.E. McLendon, D.D. Bigner, J.N. Rich, Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66, 7843–7848 (2006)
L. Ricci-Vitiani, R. Pallini, M. Biffoni, M. Todaro, G. Invernici, T. Cenci, G. Maira, E.A. Parati, G. Stassi, L.M. Larocca, R. De Maria, Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 (2010)
P. Carmeliet, R.K. Jain, Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000)
C. Grange, M. Tapparo, F. Collino, L. Vitillo, C. Damasco, M.C. Deregibus, C. Tetta, B. Bussolati, G. Camussi, Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356 (2011)
A. Conigliaro, V. Costa, A. Lo Dico, L. Saieva, S. Buccheri, F. Dieli, M. Manno, S. Raccosta, C. Mancone, M. Tripodi, G. De Leo, R. Alessandro, CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 14, 155 (2015)
E.J. Ekstrom, C. Bergenfelz, V. von Bulow, F. Serifler, E. Carlemalm, G. Jonsson, T. Andersson, K. Leandersson, WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 13, 88 (2014)
S.K. Gopal, D.W. Greening, E.G. Hanssen, H.J. Zhu, R.J. Simpson, R.A. Mathias, Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget 7, 19709–19722 (2016)
Y. Liu, F. Luo, B. Wang, H. Li, Y. Xu, X. Liu, L. Shi, X. Lu, W. Xu, L. Lu, Y. Qin, Q. Xiang, Q. Liu, STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 370, 125–135 (2016)
Y.K. Chan, H. Zhang, P. Liu, S.W. Tsao, M.L. Lung, N.K. Mak, R. Ngok-Shun Wong, P. Ying-Kit Yue, Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int. J. Cancer 137, 1830–1841 (2015)
K. Pakravan, S. Babashah, M. Sadeghizadeh, S.J. Mowla, M. Mossahebi-Mohammadi, F. Ataei, N. Dana, M. Javan, MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell. Oncol. 40, 457–470 (2017)
H. Tadokoro, T. Umezu, K. Ohyashiki, T. Hirano, J.H. Ohyashiki, Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 288, 34343–34351 (2013)
T. Umezu, H. Tadokoro, K. Azuma, S. Yoshizawa, K. Ohyashiki, J.H. Ohyashiki, Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124, 3748–3757 (2014)
R. Kalluri, R.A. Weinberg, The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009)
S. Shi, Q. Zhang, Y. Xia, B. You, Y. Shan, L. Bao, L. Li, Y. You, Z. Gu, Mesenchymal stem cell-derived exosomes facilitate nasopharyngeal carcinoma progression. Am. J. Cancer Res. 6, 459–472 (2016)
C.A. Franzen, R.H. Blackwell, V. Todorovic, K.A. Greco, K.E. Foreman, R.C. Flanigan, P.C. Kuo, G.N. Gupta, Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogene 4, e163 (2015)
M. Aga, G.L. Bentz, S. Raffa, M.R. Torrisi, S. Kondo, N. Wakisaka, T. Yoshizaki, J.S. Pagano, J. Shackelford, Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene 33, 4613–4622 (2014)
W. Qin, Y. Tsukasaki, S. Dasgupta, N. Mukhopadhyay, M. Ikebe, E.R. Sauter, Exosomes in human breast milk promote emt. Clin. Cancer Res. 22, 4517–4524 (2016)
J. Zhang, L. Ma, MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 31, 653–662 (2012)
D. Xiao, S. Barry, D. Kmetz, M. Egger, J. Pan, S.N. Rai, J. Qu, K.M. McMasters, H. Hao, Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. 376, 318–327 (2016)
I.J. Fidler, The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003)
M. Rodriguez, J. Silva, A. Herrera, M. Herrera, C. Pena, P. Martin, B. Gil-Calderon, M.J. Larriba, M.J. Coronado, B. Soldevilla, V.S. Turrion, M. Provencio, A. Sanchez, F. Bonilla, V. Garcia-Barberan, Exosomes enriched in stemness/metastatic-related mRNAS promote oncogenic potential in breast cancer. Oncotarget 6, 40575–40587 (2015)
T. Arita, D. Ichikawa, H. Konishi, S. Komatsu, A. Shiozaki, S. Ogino, Y. Fujita, H. Hiramoto, J. Hamada, K. Shoda, T. Kosuga, H. Fujiwara, K. Okamoto, E. Otsuji, Tumor exosome-mediated promotion of adhesion to mesothelial cells in gastric cancer cells. Oncotarget 7, 56855–56863 (2016)
L. Li, C. Li, S. Wang, Z. Wang, J. Jiang, W. Wang, X. Li, J. Chen, K. Liu, C. Li, G. Zhu, Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver mir-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 76, 1770–1780 (2016)
J. Liao, R. Liu, Y.J. Shi, L.H. Yin, Y.P. Pu, Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int. J. Oncol. 48, 2567–2579 (2016)
M. Yang, J. Chen, F. Su, B. Yu, F. Su, L. Lin, Y. Liu, J.-D. Huang, E. Song, Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 10, 1–13 (2011)
X.L. Bai, Q. Zhang, L.Y. Ye, F. Liang, X. Sun, Y. Chen, Q.D. Hu, Q.H. Fu, W. Su, Z. Chen, Z.P. Zhuang, T.B. Liang, Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/beta-catenin signaling. Oncogene 34, 4089–4097 (2015)
M.Y. Fong, W. Zhou, L. Liu, A.Y. Alontaga, M. Chandra, J. Ashby, A. Chow, S.T. O'Connor, S. Li, A.R. Chin, G. Somlo, M. Palomares, Z. Li, J.R. Tremblay, A. Tsuyada, G. Sun, M.A. Reid, X. Wu, P. Swiderski, X. Ren, Y. Shi, M. Kong, W. Zhong, Y. Chen, S.E. Wang, Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015)
M.M. Gottesman, Mechanisms of cancer drug resistance. Annu. Rev. Med. 53, 615–627 (2002)
W.X. Chen, X.M. Liu, M.M. Lv, L. Chen, J.H. Zhao, S.L. Zhong, M.H. Ji, Q. Hu, Z. Luo, J.Z. Wu, J.H. Tang, Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One 9, e95240 (2014)
M.M. Lv, X.Y. Zhu, W.X. Chen, S.L. Zhong, Q. Hu, T.F. Ma, J. Zhang, L. Chen, J.H. Tang, J.H. Zhao, Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 35, 10773–10779 (2014)
C.L. Au Yeung, N.N. Co, T. Tsuruga, T.L. Yeung, S.Y. Kwan, C.S. Leung, Y. Li, E.S. Lu, K. Kwan, K.K. Wong, R. Schmandt, K.H. Lu, S.C. Mok, Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 7, 11150 (2016)
Y. Hu, C. Yan, L. Mu, K. Huang, X. Li, D. Tao, Y. Wu, J. Qin, Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One 10, e0125625 (2015)
R. Ji, B. Zhang, X. Zhang, J. Xue, X. Yuan, Y. Yan, M. Wang, W. Zhu, H. Qian, W. Xu, Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle 14, 2473–2483 (2015)
T. Aung, B. Chapuy, D. Vogel, D. Wenzel, M. Oppermann, M. Lahmann, T. Weinhage, K. Menck, T. Hupfeld, R. Koch, L. Trumper, G.G. Wulf, Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl. Acad. Sci. U. S. A. 108, 15336–15341 (2011)
V. Ciravolo, V. Huber, G.C. Ghedini, E. Venturelli, F. Bianchi, M. Campiglio, D. Morelli, A. Villa, P. Della Mina, S. Menard, P. Filipazzi, L. Rivoltini, E. Tagliabue, S.M. Pupa, Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 227, 658–667 (2012)
R. Safaei, B.J. Larson, T.C. Cheng, M.A. Gibson, S. Otani, W. Naerdemann, S.B. Howell, Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 4, 1595–1604 (2005)
S. Loewer, M.N. Cabili, M. Guttman, Y.H. Loh, K. Thomas, I.H. Park, M. Garber, M. Curran, T. Onder, S. Agarwal, P.D. Manos, S. Datta, E.S. Lander, T.M. Schlaeger, G.Q. Daley, J.L. Rinn, Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 42, 1113–1117 (2010)
Y. Pan, C. Li, J. Chen, K. Zhang, X. Chu, R. Wang, L. Chen, The emerging roles of long noncoding rna ror (lincrna-ror) and its possible mechanisms in human cancers. Cell. Physiol. Biochem. 40, 219–229 (2016)
K. Takahashi, I.K. Yan, T. Kogure, H. Haga, T. Patel, Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 4, 458–467 (2014)
J. Fan, Y. Xing, X. Wen, R. Jia, H. Ni, J. He, X. Ding, H. Pan, G. Qian, S. Ge, A.R. Hoffman, H. Zhang, X. Fan, Long non-coding RNA ROR decoys gene-specific histone methylation to promote tumorigenesis. Genome Biol. 16, 139 (2015)
T.H. Cheung, T.A. Rando, Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340 (2013)
P.K. Lim, S.A. Bliss, S.A. Patel, M. Taborga, M.A. Dave, L.A. Gregory, S.J. Greco, M. Bryan, P.S. Patel, P. Rameshwar, Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 71, 1550–1560 (2011)
M. Ono, N. Kosaka, N. Tominaga, Y. Yoshioka, F. Takeshita, R.-u. Takahashi, M. Yoshida, H. Tsuda, K. Tamura, T. Ochiya, Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 7, ra63 (2014)
S.A. Bliss, G. Sinha, O. Sandiford, L. Williams, D.J. Engelberth, K. Guiro, L.L. Isenalumhe, S.J. Greco, S. Ayer, M. Bryan, R. Kumar, N. Ponzio, P. Rameshwar, Mesenchymal stem cell-derived exosomes stimulates cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 76, 5832–5844 (2016)
M. Collado, M.A. Blasco, M. Serrano, Cellular senescence in cancer and aging. Cell 130, 223–233 (2007)
P. Kahlem, B. Dorken, C.A. Schmitt, Cellular senescence in cancer treatment: friend or foe? J. Clin. Invest. 113, 169–174 (2004)
H.D. Skinner, V.C. Sandulache, T.J. Ow, R.E. Meyn, J.S. Yordy, B.M. Beadle, A.L. Fitzgerald, U. Giri, K.K. Ang, J.N. Myers, TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin. Cancer Res. 18, 290–300 (2012)
B. Jonchere, A. Vetillard, B. Toutain, D. Lam, A.C. Bernard, C. Henry, S. De Carne Trecesson, E. Gamelin, P. Juin, C. Guette, O. Coqueret, Irinotecan treatment and senescence failure promote the emergence of more transformed and invasive cells that depend on anti-apoptotic Mcl-1. Oncotarget 6, 409–426 (2015)
A.L.C. Ong, T.S. Ramasamy, Role of sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res. Rev. 43, 64–80 (2018)
X. Yu, T. Riley, A.J. Levine, The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 276, 2201–2212 (2009)
Y. Sun, W. Zheng, Z. Guo, Q. Ju, L. Zhu, J. Gao, L. Zhou, F. Liu, Y. Xu, Q. Zhan, Z. Zhou, W. Sun, X. Zhao, A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer. Sci. Rep. 6, 28083 (2016)
N. Malaquin, A. Martinez, F. Rodier, Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 82, 39–49 (2016)
J.P. Coppe, K. Kauser, J. Campisi, C.M. Beausejour, Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 281, 29568–29574 (2006)
X. Sun, M. Vale, E. Leung, J.R. Kanwar, R. Gupta, G.W. Krissansen, Mouse B7-H3 induces antitumor immunity. Gene Ther. 10, 1728–1734 (2003)
B.D. Lehmann, M.S. Paine, A.M. Brooks, J.A. McCubrey, R.H. Renegar, R. Wang, D.M. Terrian, Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 68, 7864–7871 (2008)
K. Weiner-Gorzel, E. Dempsey, M. Milewska, A. McGoldrick, V. Toh, A. Walsh, S. Lindsay, L. Gubbins, A. Cannon, D. Sharpe, J. O'Sullivan, M. Murphy, S.F. Madden, M. Kell, A. McCann, F. Furlong, Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med. 4, 745–758 (2015)
F. Furlong, P. Fitzpatrick, S. O'Toole, S. Phelan, B. McGrogan, A. Maguire, A. O'Grady, M. Gallagher, M. Prencipe, A. McGoldrick, P. McGettigan, D. Brennan, O. Sheils, C. Martin, E. W. Kay, J. O'Leary, A. McCann, Low MAD2 expression levels associate with reduced progression-free survival in patients with high-grade serous epithelial ovarian cancer. J. Pathol. 226, 746–755 (2012)
B.W. van Balkom, O.G. de Jong, M. Smits, J. Brummelman, K. den Ouden, P.M. de Bree, M.A. van Eijndhoven, D.M. Pegtel, W. Stoorvogel, T. Wurdinger, M.C. Verhaar, Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121, 3997–4006 (2013)
S. Baroni, S. Romero-Cordoba, I. Plantamura, M. Dugo, E. D'Ippolito, A. Cataldo, G. Cosentino, V. Angeloni, A. Rossini, M.G. Daidone, M.V. Iorio, Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 7, e2312 (2016)
A. Gutkin, O. Uziel, E. Beery, J. Nordenberg, M. Pinchasi, H. Goldvaser, S. Henick, M. Goldberg, M. Lahav, Tumor cells derived exosomes contain hTERT mRNA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget 7, 59173–59188 (2016)
J. Gu, H. Qian, L. Shen, X. Zhang, W. Zhu, L. Huang, Y. Yan, F. Mao, C. Zhao, Y. Shi, W. Xu, Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-beta/Smad pathway. PLoS One 7, e52465 (2012)
J. Webber, R. Steadman, M.D. Mason, Z. Tabi, A. Clayton, Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 70, 9621–9630 (2010)
A. Orimo, Y. Tomioka, Y. Shimizu, M. Sato, S. Oigawa, K. Kamata, Y. Nogi, S. Inoue, M. Takahashi, T. Hata, M. Muramatsu, Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin. Cancer Res. 7, 3097–3105 (2001)
L.M. Sobral, A. Bufalino, M.A. Lopes, E. Graner, T. Salo, R.D. Coletta, Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol. 47, 840–846 (2011)
S. Vong, R. Kalluri, The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer 2, 1139–1145 (2011)
C. Corrado, L. Saieva, S. Raimondo, A. Santoro, G. De Leo, R. Alessandro, Chronic myelogenous leukaemia exosomes modulate bone marrow microenvironment through activation of epidermal growth factor receptor. J. Cell. Mol. Med. 20, 1829–1839 (2016)
L. Wu, X. Zhang, B. Zhang, H. Shi, X. Yuan, Y. Sun, Z. Pan, H. Qian, W. Xu, Exosomes derived from gastric cancer cells activate NF-kappaB pathway in macrophages to promote cancer progression. Tumour Biol. 37, 12169–12180 (2016)
M. Egeblad, E.S. Nakasone, Z. Werb, Tumors as organs: complex tissues that interface with the entire organism. Dev. Cell 18, 884–901 (2010)
L.R. Languino, A. Singh, M. Prisco, G.J. Inman, A. Luginbuhl, J.M. Curry, A.P. South, Exosome-mediated transfer from the tumor microenvironment increases TGFbeta signaling in squamous cell carcinoma. Am. J. Transl. Res. 8, 2432–2437 (2016)
M.C. Boelens, T.J. Wu, B.Y. Nabet, B. Xu, Y. Qiu, T. Yoon, D.J. Azzam, C. Twyman-Saint Victor, B.Z. Wiemann, H. Ishwaran, P.J. Ter Brugge, J. Jonkers, J. Slingerland, A.J. Minn, Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159, 499–513 (2014)
D.J. Prockop, Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 (1997)
G. Lazennec, C. Jorgensen, Concise Review: Adult multipotent stromal cells and cancer: risk or benefit? Stem Cells 26, 1387–1394 (2008)
B. Cousin, E. Ravet, S. Poglio, F. De Toni, M. Bertuzzi, H. Lulka, I. Touil, M. Andre, J.L. Grolleau, J.M. Peron, J.P. Chavoin, P. Bourin, L. Penicaud, L. Casteilla, L. Buscail, P. Cordelier, Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One 4, e6278 (2009)
L. Qiao, Z.L. Xu, T.J. Zhao, L.H. Ye, X.D. Zhang, Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 269, 67–77 (2008)
Y. Yulyana, I.A. Ho, K.C. Sia, J.P. Newman, X.Y. Toh, B.B. Endaya, J.K. Chan, M. Gnecchi, H. Huynh, A.Y. Chung, K.H. Lim, H.S. Leong, N.G. Iyer, K.M. Hui, P.Y. Lam, Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol. Ther. 23, 746–756 (2015)
O. Attar-Schneider, V. Zismanov, L. Drucker, M. Gottfried, Secretome of human bone marrow mesenchymal stem cells: an emerging player in lung cancer progression and mechanisms of translation initiation. Tumor Biol. 37, 4755–4765 (2016)
J.K. Lee, S.R. Park, B.K. Jung, Y.K. Jeon, Y.S. Lee, M.K. Kim, Y.G. Kim, J.Y. Jang, C.W. Kim, Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8, e84256 (2013)
A.Y. Khakoo, S. Pati, S.A. Anderson, W. Reid, M.F. Elshal, I.I. Rovira, A.T. Nguyen, D. Malide, C.A. Combs, G. Hall, J. Zhang, M. Raffeld, T.B. Rogers, W. Stetler-Stevenson, J.A. Frank, M. Reitz, T. Finkel, Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J. Exp. Med. 203, 1235–1247 (2006)
J.F. Ji, B.P. He, S.T. Dheen, S.S.W. Tay, Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 22, 415–427 (2004)
B.M. Beckermann, G. Kallifatidis, A. Groth, D. Frommhold, A. Apel, J. Mattern, A.V. Salnikov, G. Moldenhauer, W. Wagner, A. Diehlmann, R. Saffrich, M. Schubert, A.D. Ho, N. Giese, M.W. Buchler, H. Friess, P. Buchler, I. Herr, VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer 99, 622–631 (2008)
A. Schmidt, D. Ladage, T. Schinköthe, U. Klausmann, C. Ulrichs, F.J. Klinz, K. Brixius, S. Arnhold, B. Desai, U. Mehlhorn, R.H.G. Schwinger, P. Staib, K. Addicks, W. Bloch, Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells 24, 1750–1758 (2006)
C. Ke, J. Chen, Y. Guo, Z.W. Chen, J. Cai, Migration mechanism of mesenchymal stem cells studied by QD/NSOM. Biochim. Biophys. Acta Biomembr. 1848, 859–868 (2015)
G. Ren, X. Zhao, Y. Wang, X. Zhang, X. Chen, C. Xu, Z.R. Yuan, A.I. Roberts, L. Zhang, B. Zheng, T. Wen, Y. Han, A.B. Rabson, J.A. Tischfield, C. Shao, Y. Shi, CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell 11, 812–824 (2012)
B.G. Cuiffo, A. Campagne, G.W. Bell, A. Lembo, F. Orso, E.C. Lien, M.K. Bhasin, M. Raimo, S.E. Hanson, A. Marusyk, D. El-Ashry, P. Hematti, K. Polyak, F. Mechta-Grigoriou, O. Mariani, S. Volinia, A. Vincent-Salomon, D. Taverna, A.E. Karnoub, MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell 15, 762–774 (2014)
A. De Boeck, P. Pauwels, K. Hensen, J.L. Rummens, W. Westbroek, A. Hendrix, D. Maynard, H. Denys, K. Lambein, G. Braems, C. Gespach, M. Bracke, O. De Wever, Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62, 550–560 (2013)
Y. Huang, P. Yu, W. Li, G. Ren, A.I. Roberts, W. Cao, X. Zhang, J. Su, X. Chen, Q. Chen, P. Shou, C. Xu, L. Du, L. Lin, N. Xie, L. Zhang, Y. Wang, Y. Shi, p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene 33, 3830–3838 (2014)
K. McLean, Y. Gong, Y. Choi, N. Deng, K. Yang, S. Bai, L. Cabrera, E. Keller, L. McCauley, K.R. Cho, R.J. Buckanovich, Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J. Clin. Invest. 121, 3206–3219 (2011)
F. Vianello, F. Villanova, V. Tisato, S. Lymperi, K.-K. Ho, A.R. Gomes, D. Marin, D. Bonnet, J. Apperley, E.W.F. Lam, F. Dazzi, Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica 95, 1081–1089 (2010)
L.Y. Lin, L.M. Du, K. Cao, Y. Huang, P.F. Yu, L.Y. Zhang, F.Y. Li, Y. Wang, Y.F. Shi, Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene 35, 6038–6042 (2016)
X. Song, Y. Ding, G. Liu, X. Yang, R. Zhao, Y. Zhang, X. Zhao, G.J. Anderson, G. Nie, Cancer Cell-derived exosomes induce mitogen-activated protein kinase-dependent monocyte survival by transport of functional receptor tyrosine kinases. J. Biol. Chem. 291, 8453–8464 (2016)
J. Choi, J. Gyamfi, H. Jang and J.S. Koo, The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 33, 133–145 (2018)
F. Leonard, L.T. Curtis, M.J. Ware, T. Nosrat, X. Liu, K. Yokoi, H.B. Frieboes, B. Godin, Macrophage polarization contributes to the anti-tumoral efficacy of mesoporous nanovectors loaded with albumin-bound paclitaxel. Front. Immunol. 8, 693 (2017)
Z. Chen, X. Feng, C.J. Herting, V. Alvarez Garcia, K. Nie, W.W. Pong, R. Rasmussen, B. Dwivedi, S. Seby, S.A. Wolf, D.H. Gutmann, D. Hambardzumyan, Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 77, 2266–2278 (2017)
M. Yin, X. Li, S. Tan, H.J. Zhou, W. Ji, S. Bellone, X. Xu, H. Zhang, A.D. Santin, G. Lou, W. Min, Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J. Clin. Invest. 126, 4157–4173 (2016)
H. Shinohara, Y. Kuranaga, M. Kumazaki, N. Sugito, Y. Yoshikawa, T. Takai, K. Taniguchi, Y. Ito, Y. Akao, Regulated polarization of tumor-associated macrophages by mir-145 via colorectal cancer–derived extracellular vesicles. J. Immunol. 199, 1505–1515 (2017)
J. Wang, K. De Veirman, S. Faict, M.A. Frassanito, D. Ribatti, A. Vacca, E. Menu, Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J. Pathol. 239, 162–173 (2016)
U. Putz, J. Howitt, A. Doan, C.-P. Goh, L.-H. Low, J. Silke, S.-S. Tan, The tumor suppressor pten is exported in exosomes and has phosphatase activity in recipient cells. Sci. Signal. 5, ra70 (2012)
A.M.M.T. Reza, Y.-J. Choi, H. Yasuda, J.-H. Kim, Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 6, 38498 (2016)
S.F. Ko, H.K. Yip, Y.Y. Zhen, C.C. Lee, C.C. Lee, C.C. Huang, S.H. Ng, J.W. Lin, Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer t-cell responses, and histopathological features. Stem Cells Int. 2015, 853506 (2015)
F. Alcayaga-Miranda, P.L. Gonzalez, A. Lopez-Verrilli, M. Varas-Godoy, C. Aguila-Diaz, L. Contreras, M. Khoury, Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget 7, 44462–44477 (2016)
H.D. Lee, B.H. Koo, Y.H. Kim, O.H. Jeon, D.S. Kim, Exosome release of ADAM15 and the functional implications of human macrophage-derived ADAM15 exosomes. FASEB J. 26, 3084–3095 (2012)
B. Costa-Silva, N.M. Aiello, A.J. Ocean, S. Singh, H. Zhang, B.K. Thakur, A. Becker, A. Hoshino, M.T. Mark, H. Molina, J. Xiang, T. Zhang, T.M. Theilen, G. Garcia-Santos, C. Williams, Y. Ararso, Y. Huang, G. Rodrigues, T.L. Shen, K.J. Labori, I.M. Lothe, E.H. Kure, J. Hernandez, A. Doussot, S.H. Ebbesen, P.M. Grandgenett, M.A. Hollingsworth, M. Jain, K. Mallya, S.K. Batra, W.R. Jarnagin, R.E. Schwartz, I. Matei, H. Peinado, B.Z. Stanger, J. Bromberg, D. Lyden, Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015)
J. Sceneay, M.J. Smyth, A. Moller, The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 32, 449–464 (2013)
H. Peinado, M. Aleckovic, S. Lavotshkin, I. Matei, B. Costa-Silva, G. Moreno-Bueno, M. Hergueta-Redondo, C. Williams, G. Garcia-Santos, C. Ghajar, A. Nitadori-Hoshino, C. Hoffman, K. Badal, B.A. Garcia, M.K. Callahan, J. Yuan, V.R. Martins, J. Skog, R.N. Kaplan, M.S. Brady, J.D. Wolchok, P.B. Chapman, Y. Kang, J. Bromberg, D. Lyden, Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012)
T. Jung, D. Castellana, P. Klingbeil, I. Cuesta Hernandez, M. Vitacolonna, D.J. Orlicky, S.R. Roffler, P. Brodt, M. Zoller, CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 11, 1093–1105 (2009)
J.L. Hood, R.S. San and S.A. Wickline, Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801 (2011)
C.A. Sanchez, E.I. Andahur, R. Valenzuela, E.A. Castellon, J.A. Fulla, C.G. Ramos, J.C. Trivino, Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 7, 3993–4008 (2016)
A. Hoshino, B. Costa-Silva, T.L. Shen, G. Rodrigues, A. Hashimoto, M. Tesic Mark, H. Molina, S. Kohsaka, A. Di Giannatale, S. Ceder, S. Singh, C. Williams, N. Soplop, K. Uryu, L. Pharmer, T. King, L. Bojmar, A.E. Davies, Y. Ararso, T. Zhang, H. Zhang, J. Hernandez, J.M. Weiss, V.D. Dumont-Cole, K. Kramer, L.H. Wexler, A. Narendran, G.K. Schwartz, J.H. Healey, P. Sandstrom, K.J. Labori, E.H. Kure, P.M. Grandgenett, M.A. Hollingsworth, M. de Sousa, S. Kaur, M. Jain, K. Mallya, S.K. Batra, W.R. Jarnagin, M.S. Brady, O. Fodstad, V. Muller, K. Pantel, A.J. Minn, M.J. Bissell, B.A. Garcia, Y. Kang, V.K. Rajasekhar, C.M. Ghajar, I. Matei, H. Peinado, J. Bromberg, D. Lyden, Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015)
S. Keller, J. Ridinger, A.K. Rupp, J.W. Janssen, P. Altevogt, Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 9, 86 (2011)
C.L. Chen, Y.F. Lai, P. Tang, K.Y. Chien, J.S. Yu, C.H. Tsai, H.W. Chen, C.C. Wu, T. Chung, C.W. Hsu, C.D. Chen, Y.S. Chang, P.L. Chang, Y.T. Chen, Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J. Proteome Res. 11, 5611–5629 (2012)
A. Kannan, R.B. Wells, S. Sivakumar, S. Komatsu, K.P. Singh, B. Samten, J.V. Philley, E.R. Sauter, M. Ikebe, S. Idell, S. Gupta, S. Dasgupta, Mitochondrial reprogramming regulates breast cancer progression. Clin. Cancer Res. 22, 3348–3360 (2016)
M.J. Donovan, M. Noerholm, S. Bentink, S. Belzer, J. Skog, V. O'Neill, J.S. Cochran, G.A. Brown, A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 18, 370–375 (2015)
M. He, H. Qin, T.C. Poon, S.C. Sze, X. Ding, N.N. Co, S.M. Ngai, T.F. Chan, N. Wong, Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis 36, 1008–1018 (2015)
G.K. Joshi, S. Deitz-McElyea, T. Liyanage, K. Lawrence, S. Mali, R. Sardar, M. Korc, Label-free nanoplasmonic-based short noncoding rna sensing at attomolar concentrations allows for quantitative and highly specific assay of microrna-10b in biological fluids and circulating exosomes. ACS Nano 9, 11075–11089 (2015)
L. Manterola, E. Guruceaga, J. Gallego Perez-Larraya, M. Gonzalez-Huarriz, P. Jauregui, S. Tejada, R. Diez-Valle, V. Segura, N. Sampron, C. Barrena, I. Ruiz, A. Agirre, A. Ayuso, J. Rodriguez, A. Gonzalez, E. Xipell, A. Matheu, A. Lopez de Munain, T. Tunon, I. Zazpe, J. Garcia-Foncillas, S. Paris, J.Y. Delattre, M.M. Alonso, A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro-Oncology 16, 520–527 (2014)
J. Skog, T. Wurdinger, S. van Rijn, D.H. Meijer, L. Gainche, W.T. Curry, B.S. Carter, A.M. Krichevsky, X.O. Breakefield, Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008)
P. Kharaziha, D. Chioureas, D. Rutishauser, G. Baltatzis, L. Lennartsson, P. Fonseca, A. Azimi, K. Hultenby, R. Zubarev, A. Ullen, J. Yachnin, S. Nilsson, T. Panaretakis, Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget 6, 21740–21754 (2015)
K. Kawakami, Y. Fujita, T. Kato, K. Mizutani, K. Kameyama, H. Tsumoto, Y. Miura, T. Deguchi, M. Ito, Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int. J. Oncol. 47, 384–390 (2015)
Y.Y. Yeh, H.G. Ozer, A.M. Lehman, K. Maddocks, L. Yu, A.J. Johnson, J.C. Byrd, Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood 125, 3297–3305 (2015)
J. Silva, V. Garcia, M. Rodriguez, M. Compte, E. Cisneros, P. Veguillas, J.M. Garcia, G. Dominguez, Y. Campos-Martin, J. Cuevas, C. Pena, M. Herrera, R. Diaz, N. Mohammed, F. Bonilla, Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosom. Cancer 51, 409–418 (2012)
N. Kosaka, H. Iguchi, Y. Yoshioka, F. Takeshita, Y. Matsuki, T. Ochiya, Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452 (2010)
M. Fabbri, A. Paone, F. Calore, R. Galli, E. Gaudio, R. Santhanam, F. Lovat, P. Fadda, C. Mao, G.J. Nuovo, N. Zanesi, M. Crawford, G.H. Ozer, D. Wernicke, H. Alder, M.A. Caligiuri, P. Nana-Sinkam, D. Perrotti, C.M. Croce, MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 109, E2110–E2116 (2012)
F. Chalmin, S. Ladoire, G. Mignot, J. Vincent, M. Bruchard, J.P. Remy-Martin, W. Boireau, A. Rouleau, B. Simon, D. Lanneau, A. De Thonel, G. Multhoff, A. Hamman, F. Martin, B. Chauffert, E. Solary, L. Zitvogel, C. Garrido, B. Ryffel, C. Borg, L. Apetoh, C. Rebe, F. Ghiringhelli, Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471 (2010)
A. Bobrie, S. Krumeich, F. Reyal, C. Recchi, L.F. Moita, M.C. Seabra, M. Ostrowski, C. Théry, Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 72, 4920–4930 (2012)
M. Ruiz-Martinez, A. Navarro, R.M. Marrades, N. Vinolas, S. Santasusagna, C. Munoz, J. Ramirez, L. Molins, M. Monzo, YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget 7, 51515–51524 (2016)
A. Bosque, L. Dietz, A. Gallego-Lleyda, M. Sanclemente, M. Iturralde, J. Naval, M.A. Alava, L. Martinez-Lostao, H.J. Thierse, A. Anel, Comparative proteomics of exosomes secreted by tumoral Jurkat T cells and normal human T cell blasts unravels a potential tumorigenic role for valosin-containing protein. Oncotarget 7, 29287–29305 (2016)
C. Federici, F. Petrucci, S. Caimi, A. Cesolini, M. Logozzi, M. Borghi, S. D'Ilio, L. Lugini, N. Violante, T. Azzarito, C. Majorani, D. Brambilla, S. Fais, Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One 9, e88193 (2014)
X.Q. Li, J.T. Liu, L.L. Fan, Y. Liu, L. Cheng, F. Wang, H.Q. Yu, J. Gao, W. Wei, H. Wang, G.P. Sun, Exosomes derived from gefitinib-treated EGFR-mutant lung cancer cells alter cisplatin sensitivity via up-regulating autophagy. Oncotarget 7, 24585–24595 (2016)
H.G. Zhang, H. Kim, C. Liu, S. Yu, J. Wang, W.E. Grizzle, R.P. Kimberly, S. Barnes, Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim. Biophys. Acta 1773, 1116–1123 (2007)
T.S. Ramasamy, A.Z. Ayob, H.H. Myint, S. Thiagarajah, F. Amini, Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 15, 96 (2015)
S. Amigorena, Cancer immunotherapy using dendritic cell-derived exosomes. Medicina (B Aires) 60 Suppl 2, 51–54 (2000)
G.G. Romagnoli, B.B. Zelante, P.A. Toniolo, I.K. Migliori, J.A.M. Barbuto, Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol. 5, 692 (2014)
Q. Rao, B. Zuo, Z. Lu, X. Gao, A. You, C. Wu, Z. Du, H. Yin, Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and human in vitro. Hepatology 64, 456–472 (2016)
J. Wang, L. Wang, Z. Lin, L. Tao, M. Chen, More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol. Med. Rep. 9, 125–131 (2014)
M. Damo, D.S. Wilson, E. Simeoni, J.A. Hubbell, TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci. Rep. 5, 17622 (2015)
Y. Xie, O. Bai, H. Zhang, J. Yuan, S. Zong, R. Chibbar, K. Slattery, M. Qureshi, Y. Wei, Y. Deng, J. Xiang, Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J. Cell. Mol. Med. 14, 2655–2666 (2010)
L.H. Lv, Y.L. Wan, Y. Lin, W. Zhang, M. Yang, G.L. Li, H.M. Lin, C.Z. Shang, Y.J. Chen, J. Min, Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 287, 15874–15885 (2012)
G. Fuhrmann, A. Serio, M. Mazo, R. Nair, M.M. Stevens, Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 205, 35–44 (2015)
F. Aqil, H. Kausar, A.K. Agrawal, J. Jeyabalan, A.H. Kyakulaga, R. Munagala, R. Gupta, Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 101, 12–21 (2016)
M.S. Kim, M.J. Haney, Y. Zhao, V. Mahajan, I. Deygen, N.L. Klyachko, E. Inskoe, A. Piroyan, M. Sokolsky, O. Okolie, S.D. Hingtgen, A.V. Kabanov, E.V. Batrakova, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12, 655–664 (2016)
R. Munagala, F. Aqil, J. Jeyabalan, R.C. Gupta, Bovine milk-derived exosomes for drug delivery. Cancer Lett. 371, 48–61 (2016)
S.C. Jang, O.Y. Kim, C.M. Yoon, D.S. Choi, T.Y. Roh, J. Park, J. Nilsson, J. Lotvall, Y.K. Kim, Y.S. Gho, Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7, 7698–7710 (2013)
K. O'Brien, M.C. Lowry, C. Corcoran, V.G. Martinez, M. Daly, S. Rani, W.M. Gallagher, M.W. Radomski, R.A. MacLeod, L. O'Driscoll, miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget 6, 32774–32789 (2015)
S. Ohno, M. Takanashi, K. Sudo, S. Ueda, A. Ishikawa, N. Matsuyama, K. Fujita, T. Mizutani, T. Ohgi, T. Ochiya, N. Gotoh, M. Kuroda, Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191 (2013)
K.A. Greco, C.A. Franzen, K.E. Foreman, R.C. Flanigan, P.C. Kuo, G.N. Gupta, PLK-1 silencing in bladder cancer by sirna delivered with exosomes. Urology 91, e241–e247 (2016)
J.L. Munoz, S.A. Bliss, S.J. Greco, S.H. Ramkissoon, K.L. Ligon, P. Rameshwar, Delivery of functional anti-mir-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther. Nucleic Acids 2, e126 (2013)
H. Qi, C. Liu, L. Long, Y. Ren, S. Zhang, X. Chang, X. Qian, H. Jia, J. Zhao, J. Sun, X. Hou, X. Yuan, C. Kang, Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 10, 3323–3333 (2016)
Y. Tian, S. Li, J. Song, T. Ji, M. Zhu, G.J. Anderson, J. Wei, G. Nie, A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390 (2014)
T. Yang, P. Martin, B. Fogarty, A. Brown, K. Schurman, R. Phipps, V.P. Yin, P. Lockman, S. Bai, Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32, 2003–2014 (2015)
C.S. Hong, L. Muller, M. Boyiadzis, T.L. Whiteside, Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One 9, e103310 (2014)
C.J. Beckham, J. Olsen, P.N. Yin, C.H. Wu, H.J. Ting, F.K. Hagen, E. Scosyrev, E.M. Messing, Y.F. Lee, Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J. Urol. 192, 583–592 (2014)
C. Berrondo, J. Flax, V. Kucherov, A. Siebert, T. Osinski, A. Rosenberg, C. Fucile, S. Richheimer, C.J. Beckham, Expression of the long non-coding rna hotair correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS One 11, e0147236 (2016)
C. Eichelser, I. Stuckrath, V. Muller, K. Milde-Langosch, H. Wikman, K. Pantel, H. Schwarzenbach, Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 5, 9650–9663 (2014)
S. Khan, H.F. Bennit, D. Turay, M. Perez, S. Mirshahidi, Y. Yuan, N.R. Wall, Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer 14, 176 (2014)
I. Vardaki, S. Ceder, D. Rutishauser, G. Baltatzis, T. Foukakis, T. Panaretakis, Periostin is identified as a putative metastatic marker in breast cancer-derived exosomes. Oncotarget 7, 74966–74978 (2016)
J. Liu, H. Sun, X. Wang, Q. Yu, S. Li, X. Yu, W. Gong, Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int. J. Mol. Sci. 15, 758–773 (2014)
T. Matsumura, K. Sugimachi, H. Iinuma, Y. Takahashi, J. Kurashige, G. Sawada, M. Ueda, R. Uchi, H. Ueo, Y. Takano, Y. Shinden, H. Eguchi, H. Yamamoto, Y. Doki, M. Mori, T. Ochiya, K. Mimori, Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br. J. Cancer 113, 275–281 (2015)
H. Ogata-Kawata, M. Izumiya, D. Kurioka, Y. Honma, Y. Yamada, K. Furuta, T. Gunji, H. Ohta, H. Okamoto, H. Sonoda, M. Watanabe, H. Nakagama, J. Yokota, T. Kohno, N. Tsuchiya, Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One 9, e92921 (2014)
Q. Li, Y. Shao, X. Zhang, T. Zheng, M. Miao, L. Qin, B. Wang, G. Ye, B. Xiao, J. Guo, Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 36, 2007–2012 (2015)
H. Wang, L. Hou, A. Li, Y. Duan, H. Gao, X. Song, Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed. Res. Int. 2014, 864894 (2014)
K. Sugimachi, T. Matsumura, H. Hirata, R. Uchi, M. Ueda, H. Ueo, Y. Shinden, T. Iguchi, H. Eguchi, K. Shirabe, T. Ochiya, Y. Maehara, K. Mimori, Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer 112, 532–538 (2015)
J. Wang, Y. Zhou, J. Lu, Y. Sun, H. Xiao, M. Liu, L. Tian, Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med. Oncol. 31, 148 (2014)
M. Guan, X. Chen, Y. Ma, L. Tang, L. Guan, X. Ren, B. Yu, W. Zhang, B. Su, MDA-9 and GRP78 as potential diagnostic biomarkers for early detection of melanoma metastasis. Tumour Biol. 36, 2973–2982 (2015)
E. Alegre, M.F. Sanmamed, C. Rodriguez, O. Carranza, S. Martin-Algarra, A. Gonzalez, Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Arch. Pathol. Lab. Med. 138, 828–832 (2014)
J. Klibi, T. Niki, A. Riedel, C. Pioche-Durieu, S. Souquere, E. Rubinstein, S. Le Moulec, J. Guigay, M. Hirashima, F. Guemira, D. Adhikary, J. Mautner, P. Busson, Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 113, 1957–1966 (2009)
Y. Li, Y. Zhang, F. Qiu, Z. Qiu, Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis 32, 1976–1983 (2011)
Y. Tanaka, H. Kamohara, K. Kinoshita, J. Kurashige, T. Ishimoto, M. Iwatsuki, M. Watanabe, H. Baba, Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 119, 1159–1167 (2013)
D.D. Taylor and C. Gercel-Taylor, MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 (2008)
X. Ying, Q. Wu, X. Wu, Q. Zhu, X. Wang, L. Jiang, X. Chen and X. Wang, Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget 7, 43076–43087 (2016)
C. Kahlert, S.A. Melo, A. Protopopov, J. Tang, S. Seth, M. Koch, J. Zhang, J. Weitz, L. Chin, A. Futreal, R. Kalluri, Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 289, 3869–3875 (2014)
S.A. Melo, L.B. Luecke, C. Kahlert, A.F. Fernandez, S.T. Gammon, J. Kaye, V.S. LeBleu, E.A. Mittendorf, J. Weitz, N. Rahbari, C. Reissfelder, C. Pilarsky, M.F. Fraga, D. Piwnica-Worms, R. Kalluri, Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015)
P.J. Mitchell, J. Welton, J. Staffurth, J. Court, M.D. Mason, Z. Tabi, A. Clayton, Can urinary exosomes act as treatment response markers in prostate cancer? J. Transl. Med. 7, 4 (2009)
T. Kato, K. Mizutani, K. Kameyama, K. Kawakami, Y. Fujita, K. Nakane, Y. Kanimoto, H. Ehara, H. Ito, M. Seishima, T. Deguchi, M. Ito, Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol. Oncol. 33, e315–e320 (2015)
X. Huang, T. Yuan, M. Liang, M. Du, S. Xia, R. Dittmar, D. Wang, W. See, B.A. Costello, F. Quevedo, W. Tan, D. Nandy, G.H. Bevan, S. Longenbach, Z. Sun, Y. Lu, T. Wang, S.N. Thibodeau, L. Boardman, M. Kohli, L. Wang, Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 67, 33–41 (2015)
James Graham Brown Cancer Center. Phase I clinical trial investigating the ability of plant exosomes to deliver curcumin to normal and malignant colon tissue. http://clinicaltrials.gov/show/NCT01294072. Accessed 16 September 2016
James Graham Brown Cancer Center. preliminary clinical trial investigating the ability of plant exosomes to abrogate oral mucositis induced by combined chemotherapy and radiation in head and neck cancer patients. http://clinicaltrials.gov/show/NCT01668849. Accessed 16 September 2016
B. Besse, M. Charrier, V. Lapierre, E. Dansin, O. Lantz, D. Planchard, T. Le Chevalier, A. Livartoski, F. Barlesi, A. Laplanche, S. Ploix, N. Vimond, I. Peguillet, C. Thery, L. Lacroix, I. Zoernig, K. Dhodapkar, M. Dhodapkar, S. Viaud, J.C. Soria, K.S. Reiners, E. Pogge von Strandmann, F. Vely, S. Rusakiewicz, A. Eggermont, J.M. Pitt, L. Zitvogel, N. Chaput, Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5, e1071008 (2016)
S. Viaud, M. Terme, C. Flament, J. Taieb, F. Andre, S. Novault, B. Escudier, C. Robert, S. Caillat-Zucman, T. Tursz, L. Zitvogel, N. Chaput, Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 4, e4942 (2009)
B. Escudier, T. Dorval, N. Chaput, F. Andre, M.P. Caby, S. Novault, C. Flament, C. Leboulaire, C. Borg, S. Amigorena, C. Boccaccio, C. Bonnerot, O. Dhellin, M. Movassagh, S. Piperno, C. Robert, V. Serra, N. Valente, J.B. Le Pecq, A. Spatz, O. Lantz, T. Tursz, E. Angevin, L. Zitvogel, Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med. 3, 10 (2005)
Exosome Diagnostics, Inc. Clinical validation of a urinary exosome gene signature in men presenting for suspicion of prostate cancer. http://www.clinicaltrials.gov/show/NCT02702856. Accessed 16 September 2016
Hospital Miguel Servet. Circulating exosomes as potential prognostic and predictive biomarkers in advanced gastric cancer patients. http://www.clinicaltrials.gov/show/NCT01779583. Accessed 16 September 2016
New Mexico Cancer Care Alliance. An observational, single-institution pilot/feasibility study of exosome testing as a screening modality for human papillomavirus-positive oropharyngeal squamous cell carcinoma. http://clinicaltrials.gov/show/NCT02147418. Accessed 16 September 2016
Centre Georges Francois Leclerc. Pilot study with the aim to quantify a stress protein in the blood and in the urine for early diagnosis of malgnant solid tumors. http://clinicaltrials.gov/show/NCT02662621. Accessed 16 September 2016
National Taiwan University Hospital. Anaplastic thyroid cancer and follicular thyroid cancer-derived exosomal analysis via treatment of lovastatin and vildagliptin and pilot prognostic study via urine exosomal biological markers in thyroid cancer patients. http://clinicaltrials.gov/show/NCT02862470. Accessed 16 September 2016
Thomas Jefferson University. Phase 1 study in humans evaluating the safety of rectus sheath implantation of diffusion chambers encapsulating autologous malignant glioma cells treated with insulin-like growth factor receptor-1 antisense oligodeoxynucleotide in 12 patients with recurrent malignant glioma. http://www.clinicaltrials.gov/show/NCT01550523. Accessed 16 September 2016
Thomas Jefferson University. Phase I study in humans evaluating the safety of rectus sheath implantation of diffusion chambers encapsulating autologous malignant glioma cells treated with insulin-like growth factor receptor-1 antisense oligodeoxynucleotide (igf-1r/as odn) in 32 patients with newly diagnosed malignant glioma. http://clinicaltrials.gov/show/NCT02507583. Accessed 16 September 2016
S. Dai, D. Wei, Z. Wu, X. Zhou, X. Wei, H. Huang, G. Li, I. Phase, clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 16, 782–790 (2008)
Centre Hospitalier Universitaire de Nice. Pilot study of exosomes before and after braf inhibitor therapy in patients with advanced unresectable or metastatic braf mutation-positive melanoma. http://www.clinicaltrials.gov/show/NCT02310451. Accessed 16 September 2016
Midwest Biomedical Research Foundation. Evaluation of microrna expression in blood and cytology specimens as a novel method for detecting barrett's esophagus. http://www.clinicaltrials.gov/show/NCT02464930. Accessed 16 September 2016
Xinqiao Hospital of Chongqing. Clinical research for the consistency analysis of pd-l1 in cancer tissue and plasma exosome. http://www.clinicaltrials.gov/show/NCT02890849. Accessed 16 September 2016
Xinqiao Hospital of Chongqing. Clinical research for the consistency analysis of pd-l1 in lung cancer tissue and plasma exosome before and after radiotherapy. http://www.clinicaltrials.gov/show/NCT02869685. Accessed 16 September 2016
Memorial Sloan Kettering Cancer Center. Interrogation of exosome-mediated intercellular signaling in patients with pancreatic cancer. http://www.clinicaltrials.gov/show/NCT02393703. Accessed 16 September 2016
Centre Oscar Lambret. Early biomarkers of tumor response in high dose hypofractionated radiotherapy word package 3: immune response. http://clinicaltrials.gov/show/NCT02439008. Accessed 16 September 2016
Acknowledgements
This work was supported by University of Malaya Programme Grant RP032-14HTM. The authors would like to thank Miss Yew Hong Wen, from Stem Cell Biology Laboratory, for her assistance in preparing the artwork and figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sundararajan, V., Sarkar, F.H. & Ramasamy, T.S. The versatile role of exosomes in cancer progression: diagnostic and therapeutic implications. Cell Oncol. 41, 223–252 (2018). https://doi.org/10.1007/s13402-018-0378-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-018-0378-4