Abstract
Background
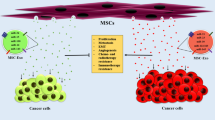
Human mesenchymal stem cells (MSCs) have been shown to be involved in the formation and modulation of tumor stroma and in interacting with tumor cells, partly through their secretome. Exosomes are nano-sized intraluminal multi-vesicular bodies secreted by most types of cells and have been found to mediate intercellular communication through the transfer of genetic information via coding and non-coding RNAs to recipient cells. Since exosomes are considered as protective and enriched sources of shuttle microRNAs (miRNAs), we hypothesized that exosomal transfer of miRNAs from MSCs may affect tumor cell behavior, particularly angiogenesis.
Methods
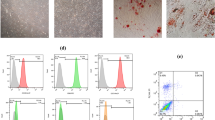
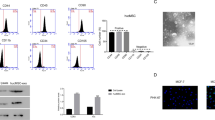
Exosomes derived from MSCs were isolated and characterized by scanning electron microscopy analyses, dynamic light scattering measurements, and Western blotting. Fold changes in miR-100 expression levels were calculated in exosomes and their corresponding donor cells by qRT-PCR. The effects of exosomal transfer of miR-100 from MSCs were assessed by qRT-PCR and Western blotting of the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. The quantification of secreted VEGF protein was determined by enzyme-linked immunosorbent assay. The putative paracrine effects of MSC-derived exosomes on tumor angiogenesis were explored by in vitro angiogenesis assays including endothelial cell proliferation, migration and tube formation assays.
Results
We found that MSC-derived exosomes induce a significant and dose-dependent decrease in the expression and secretion of vascular endothelial growth factor (VEGF) through modulating the mTOR/HIF-1α signaling axis in breast cancer-derived cells. We also found that miR-100 is enriched in MSC-derived exosomes and that its transfer to breast cancer-derived cells is associated with the down-regulation of VEGF in a time-dependent manner. The putative role of exosomal miR-100 transfer in regulating VEGF expression was substantiated by the ability of anti-miR-100 to rescue the inhibitory effects of MSC-derived exosomes on the expression of VEGF in breast cancer-derived cells. In addition, we found that down-regulation of VEGF mediated by MSC-derived exosomes can affect the vascular behavior of endothelial cells in vitro.
Conclusions
Overall, our findings suggest that exosomal transfer of miR-100 may be a novel mechanism underlying the paracrine effects of MSC-derived exosomes and may provide a means by which these vesicles can modulate vascular responses within the microenvironment of breast cancer cells.






Similar content being viewed by others
References
S.B. Fox, D.G. Generali, A.L. Harris, Breast tumour angiogenesis. Breast Cancer Res 9, 216 (2007). doi:10.1186/bcr1796
R. Sharma, T.P. Khaket, C. Dutta, B. Chakraborty, T.K. Mukherjee, Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol 40, 199–208 (2017). doi:10.1007/s13402-017-0324-x
R. Paduch, The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol 39, 397–410 (2016). doi:10.1007/s13402-016-0281-9
A. Hoeben, B. Landuyt, M.S. Highley, H. Wildiers, A.T. Van Oosterom, E.A. De Bruijn, Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56, 549–580 (2004). doi:10.1124/pr.56.4.3
J.A. Forsythe, B.H. Jiang, N.V. Iyer, F. Agani, S.W. Leung, R.D. Koos, G.L. Semenza, Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16, 4604–4613 (1996)
A. Fasolo, C. Sessa, mTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs 17, 1717–1734 (2008). doi:10.1517/13543784.17.11.1717
R. Humar, F.N. Kiefer, H. Berns, T.J. Resink, E.J. Battegay, Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J 16, 771–780 (2002). doi:10.1096/fj.01-0658com
H. Populo, J.M. Lopes, P. Soares, The mTOR signalling pathway in human cancer. Int J Mol Sci 13, 1886–1918 (2012). doi:10.3390/ijms13021886
H. Zhong, K. Chiles, D. Feldser, E. Laughner, C. Hanrahan, M.M. Georgescu, J.W. Simons, G.L. Semenza, Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res 60, 1541–1545 (2000)
D. Del Bufalo, L. Ciuffreda, D. Trisciuoglio, M. Desideri, F. Cognetti, G. Zupi, M. Milella, Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res 66, 5549–5554 (2006). doi:10.1158/0008-5472.CAN-05-2825
S.C. Land, A.R. Tee, Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 282, 20534–20543 (2007). doi:10.1074/jbc.M611782200
A. Uccelli, L. Moretta, V. Pistoia, Mesenchymal stem cells in health and disease. Nat Rev Immunol 8, 726–736 (2008). doi:10.1038/nri2395
Y. Wang, X. Chen, W. Cao, Y. Shi, Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol 15, 1009–1016 (2014). doi:10.1038/ni.3002
G. Ren, X. Chen, F. Dong, W. Li, X. Ren, Y. Zhang, Y. Shi, Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem Cells Transl Med 1, 51–58 (2012). doi:10.5966/sctm.2011-0019
S.A. Bergfeld, Y.A. DeClerck, Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 29, 249–261 (2010). doi:10.1007/s10555-010-9222-7
B.D. Roorda, A. ter Elst, W.A. Kamps, E.S. de Bont, Bone marrow-derived cells and tumor growth: Contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit Rev Oncol Hematol 69, 187–198 (2009). doi:10.1016/j.critrevonc.2008.06.004
R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes. Semin Cell Dev Biol 40, 82–88 (2015). doi:10.1016/j.semcdb.2015.03.001
H. Valadi, K. Ekstrom, A. Bossios, M. Sjostrand, J.J. Lee, J.O. Lotvall, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9, 654–659 (2007). doi:10.1038/ncb1596
Y. Lee, S. El Andaloussi, M.J. Wood, Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 21, R125–R134 (2012). doi:10.1093/hmg/dds317
T.S. Chen, R.C. Lai, M.M. Lee, A.B. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 38, 215–224 (2010). doi:10.1093/nar/gkp857
S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Perez Lanzon, N. Zini, B. Naaijkens, F. Perut, H.W. Niessen, N. Baldini, D.M. Pegtel, Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6, 127 (2015). doi:10.1186/s13287-015-0116-z
M.D. Jansson, A.H. Lund, MicroRNA and cancer. Mol Oncol 6, 590–610 (2012). doi:10.1016/j.molonc.2012.09.006
S. Babashah, M. Soleimani, The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer 47, 1127–1137 (2011)
S. Babashah, MicroRNAs: Key Regulators of Oncogenesis (Springer International Publishing, Switzerland, 2014)
I. Fkih M'hamed, M. Privat, F. Ponelle, F. Penault-Llorca, A. Kenani, Y.J. Bignon, Identification of miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative breast cancer biomarkers. Cell Oncol 38, 433–442 (2015). doi:10.1007/s13402-015-0239-3
D. Chen, Y. Sun, Y. Yuan, Z. Han, P. Zhang, J. Zhang, M.J. You, J. Teruya-Feldstein, M. Wang, S. Gupta, M.C. Hung, H. Liang, L. Ma, miR-100 induces epithelial-mesenchymal transition but suppresses tumorigenesis, migration and invasion. PLoS Genet 10, e1004177–e1002014. doi:10.1371/journal.pgen.1004177
C.A. Gebeshuber, J. Martinez, miR-100 suppresses IGF2 and inhibits breast tumorigenesis by interfering with proliferation and survival signaling. Oncogene 32, 3306–3310 (2013). doi:10.1038/onc.2012.372
L. Deng, L. Shang, S. Bai, J. Chen, X. He, R. Martin-Trevino, S. Chen, X.Y. Li, X. Meng, B. Yu, X. Wang, Y. Liu, S.P. McDermott, A.E. Ariazi, C. Ginestier, I. Ibarra, J. Ke, T. Luther, S.G. Clouthier, L. Xu, G. Shan, E. Song, H. Yao, G.J. Hannon, S.J. Weiss, M.S. Wicha, S. Liu, MicroRNA100 inhibits self-renewal of breast cancer stem-like cells and breast tumor development. Cancer Res 74, 6648–6660 (2014). doi:10.1158/0008-5472.CAN-13-3710
A. Petrelli, R. Carollo, M. Cargnelutti, F. Iovino, M. Callari, D. Cimino, M. Todaro, L.R. Mangiapane, A. Giammona, A. Cordova, F. Montemurro, D. Taverna, M.G. Daidone, G. Stassi, S. Giordano, By promoting cell differentiation, miR-100 sensitizes basal-like breast cancer stem cells to hormonal therapy. Oncotarget 6, 2315–2330 (2015). doi:10.18632/oncotarget.2962
W.L. Ng, D. Yan, X. Zhang, Y.Y. Mo, Y. Wang, Over-expression of miR-100 is responsible for the low-expression of ATM in the human glioma cell line: M059J. DNA Repair 9, 1170–1175 (2010). doi:10.1016/j.dnarep.2010.08.007
N. Kosaka, H. Iguchi, Y. Yoshioka, F. Takeshita, Y. Matsuki, T. Ochiya, Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285, 17442–17452 (2010). doi:10.1074/jbc.M110.107821
S. Ohno, M. Takanashi, K. Sudo, S. Ueda, A. Ishikawa, N. Matsuyama, K. Fujita, T. Mizutani, T. Ohgi, T. Ochiya, N. Gotoh, M. Kuroda, Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21, 185–191 (2012). doi:10.1038/mt.2012.180
A. Caivano, F. La Rocca, V. Simeon, M. Girasole, S. Dinarelli, I. Laurenzana, A. De Stradis, L. De Luca, S. Trino, A. Traficante, G. D'Arena, G. Mansueto, O. Villani, G. Pietrantuono, L. Laurenti, L. Del Vecchio, P. Musto, MicroRNA-155 in serum-derived extracellular vesicles as a potential biomarker for hematologic malignancies - a short report. Cell Oncol 40, 97–103 (2017). doi:10.1007/s13402-016-0300-x
J.A. Potian, H. Aviv, N.M. Ponzio, J.S. Harrison, P. Rameshwar, Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 171, 3426–3434 (2003)
C. Thery, S. Amigorena, G. Raposo,A. Clayton, Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell. Biol. Chapter 3, Unit 3 22 (2006) doi:10.1002/0471143030.cb0322s30
L. Chen, Y. Wang, Y. Pan, L. Zhang, C. Shen, G. Qin, M. Ashraf, N. Weintraub, G. Ma, Y. Tang, Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431, 566–571 (2013). doi:10.1016/j.bbrc.2013.01.015
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25, 402–408 (2001). doi:10.1006/meth.2001.1262
F. Collino, M.C. Deregibus, S. Bruno, L. Sterpone, G. Aghemo, L. Viltono, C. Tetta, G. Camussi, Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 5, e11803 (2010). doi:10.1371/journal.pone.0011803
D.J. Hicklin, L.M. Ellis, Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23, 1011–1027 (2005). doi:10.1200/JCO.2005.06.081
Y. Wu, A.T. Hooper, Z. Zhong, L. Witte, P. Bohlen, S. Rafii, D.J. Hicklin, The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer 119, 1519–1529 (2006). doi:10.1002/ijc.21865
A. Shibata, T. Nagaya, T. Imai, H. Funahashi, A. Nakao, H. Seo, Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat 73, 237–243 (2002)
T.H. Lee, S. Seng, M. Sekine, C. Hinton, Y. Fu, H.K. Avraham, S. Avraham, Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med 4, e186 (2007). doi:10.1371/journal.pmed.0040186
E. Maj, D. Papiernik, J. Wietrzyk, Antiangiogenic cancer treatment: The great discovery and greater complexity (review). Int J Oncol 49, 1773–1784 (2016). doi:10.3892/ijo.2016.3709
Y. Liang, R.A. Brekken, S.M. Hyder, Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer 13, 905–919 (2006). doi:10.1677/erc.1.012211
L.S. Steelman, A.M. Martelli, L. Cocco, M. Libra, F. Nicoletti, S.L. Abrams, J.A. McCubrey, The therapeutic potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol 82, 1189–1212 (2016). doi:10.1111/bcp.12958
M.S. Rotundo, T. Galeano, P. Tassone, P. Tagliaferri, mTOR inhibitors, a new era for metastatic luminal HER2-negative breast cancer? A systematic review and a meta-analysis of randomized trials. Oncotarget 7, 27055–27066 (2016). doi:10.18632/oncotarget.7446
S. Grundmann, F.P. Hans, S. Kinniry, J. Heinke, T. Helbing, F. Bluhm, J.P. Sluijter, I. Hoefer, G. Pasterkamp, C. Bode, M. Moser, MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation 123, 999–1009 (2011). doi:10.1161/CIRCULATIONAHA.110.000323
Y. Jin, S.D. Tymen, D. Chen, Z.J. Fang, Y. Zhao, D. Dragas, Y. Dai, P.T. Marucha, X. Zhou, MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One 8, e64434 (2013). doi:10.1371/journal.pone.0064434
A.K. Nagaraja, C.J. Creighton, Z. Yu, H. Zhu, P.H. Gunaratne, J.G. Reid, E. Olokpa, H. Itamochi, N.T. Ueno, S.M. Hawkins, M.L. Anderson, M.M. Matzuk, A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 24, 447–463 (2010). doi:10.1210/me.2009-0295
N. Zhang, H. Fu, L. Song, Y. Ding, X. Wang, C. Zhao, Y. Zhao, F. Jiao, MicroRNA-100 promotes migration and invasion through mammalian target of rapamycin in esophageal squamous cell carcinoma. Oncol Rep 32, 1409–1418 (2014). doi:10.3892/or.2014.3389
S.M. Weis, D.A. Cheresh, Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat Med 17, 1359–1370 (2011). doi:10.1038/nm.2537
P.N. Bernatchez, S. Soker, M.G. Sirois, Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem 274, 31047–31054 (1999)
S. Wang, X. Li, M. Parra, E. Verdin, R. Bassel-Duby, E.N. Olson, Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A 105, 7738–7743 (2008). doi:10.1073/pnas.0802857105
M. Galie, G. Konstantinidou, D. Peroni, I. Scambi, C. Marchini, V. Lisi, M. Krampera, P. Magnani, F. Merigo, M. Montani, F. Boschi, P. Marzola, R. Orru, P. Farace, A. Sbarbati, A. Amici, Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene 27, 2542–2551 (2008). doi:10.1038/sj.onc.1210920
A.E. Karnoub, A.B. Dash, A.P. Vo, A. Sullivan, M.W. Brooks, G.W. Bell, A.L. Richardson, K. Polyak, R. Tubo, R.A. Weinberg, Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007). doi:10.1038/nature06188
X.L. Yan, C.J. Fu, L. Chen, J.H. Qin, Q. Zeng, H.F. Yuan, X. Nan, H.X. Chen, J.N. Zhou, Y.L. Lin, X.M. Zhang, C.Z. Yu, W. Yue, X.T. Pei, Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat 132, 153–164 (2012). doi:10.1007/s10549-011-1577-0
A.Y. Khakoo, S. Pati, S.A. Anderson, W. Reid, M.F. Elshal, A.T. Rovira II, D. Nguyen, C.A. Malide, G. Combs, J. Hall, M. Zhang, T.B. Raffeld, W. Rogers, J.A. Stetler-Stevenson, M. Frank, T.F. Reitz, Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med 203, 1235–1247 (2006). doi:10.1084/jem.20051921
B. Cousin, E. Ravet, S. Poglio, F. De Toni, M. Bertuzzi, H. Lulka, I. Touil, M. Andre, J.L. Grolleau, J.M. Peron, J.P. Chavoin, P. Bourin, L. Penicaud, L. Casteilla, L. Buscail, P. Cordelier, Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One 4, e6278 (2009). doi:10.1371/journal.pone.0006278
P. Secchiero, S. Zorzet, C. Tripodo, F. Corallini, E. Melloni, L. Caruso, R. Bosco, S. Ingrao, B. Zavan, G. Zauli, Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One 5, e11140 (2010). doi:10.1371/journal.pone.0011140
A.H. Klopp, A. Gupta, E. Spaeth, M. Andreeff, F. Marini 3rd, Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells 29, 11–19 (2011). doi:10.1002/stem.559
W. Zhu, L. Huang, Y. Li, X. Zhang, J. Gu, Y. Yan, X. Xu, M. Wang, H. Qian, W. Xu, Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315, 28–37 (2012). doi:10.1016/j.canlet.2011.10.002
J.K. Lee, S.R. Park, B.K. Jung, Y.K. Jeon, Y.S. Lee, M.K. Kim, Y.G. Kim, J.Y. Jang, C.W. Kim, Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8, e84256 (2013). doi:10.1371/journal.pone.0084256
A.P. Wang, X.H. Li, S.X. Gong, W.Q. Li, C.P. Hu, Z. Zhang, Y.J. Li, miR-100 suppresses mTOR signaling in hypoxia-induced pulmonary hypertension in rats. Eur. J. Pharmacol 765, 565–573 (2015). doi:10.1016/j.ejphar.2015.09.031
B. Zhang, R. Zhao, Y. He, X. Fu, L. Fu, Z. Zhu, J.T. Dong, MicroRNA 100 sensitizes luminal A breast cancer cells to paclitaxel treatment in part by targeting mTOR. Oncotarget 7, 5702–5714 (2016). doi:10.18632/oncotarget.6790
K.M. Dodd, J. Yang, M.H. Shen, J.R. Sampson, A.R. Tee, mTORC1 drives HIF-1alpha and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 34, 2239–2250 (2015). doi:10.1038/onc.2014.164
Acknowledgements
The authors are grateful to the members of the Departments of Genetics and Biochemistry, Tarbiat Modares University, for their excellent technical assistance and advice. This work was supported by research grants from the Tarbiat Modares University and the Council for Development of Stem Cell Sciences and Technologies (Grant No. 11/77227).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
ESM 1
(PPTX 1401 kb)
Rights and permissions
About this article
Cite this article
Pakravan, K., Babashah, S., Sadeghizadeh, M. et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol. 40, 457–470 (2017). https://doi.org/10.1007/s13402-017-0335-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0335-7