Abstract
Exosomes are nano-sized membrane vesicles secreted by both normal and cancer cells. Emerging evidence indicates that cancer cells derived exosomes contribute to cancer progression through the modulation of tumor microenvironment. However, the effects of exosomes derived from gastric cancer cells on macrophages are not well understood. In this study, we investigated the biological role of gastric cancer cells derived exosomes in the activation of macrophages. We demonstrated that gastric cancer cells derived exosomes activated macrophages to express increased levels of proinflammatory factors, which in turn promoted tumor cell proliferation and migration. In addition, gastric cancer cells derived exosomes remarkably upregulated the phosphorylation of NF-κB in macrophages. Inhibiting the activation of NF-κB reversed the upregulation of proinflammatory factors in macrophages and blocked their promoting effects on gastric cancer cells. Moreover, we found that gastric cancer cells derived exosomes could also activate macrophages from human peripheral blood monocytes through the activation of NF-κB. In conclusion, our results suggest that gastric cancer cells derived exosomes stimulate the activation of NF-κB pathway in macrophages to promote cancer progression, which provides a potential therapeutic approach for gastric cancer by interfering with the interaction between exosomes and macrophages in tumor microenvironment.






Similar content being viewed by others
References
Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535–47.
Kim HS, Kim SO, Kim BS. Use of a clinical pathway in laparoscopic gastrectomy for gastric cancer. World J Gastroenterol. 2015;21(48):13507–17.
Zhang X, Yuan X, Shi H, Wu LJ, Qian H, Xu WR. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83–96.
Zhang W, Peng P, Kuang Y, Yang J, Cau D, You Y, et al. Characterization of exosomes derived from ovarian cancer cells and normal ovarian epithelial cells by nanoparticle tracking analysis. Tumour Biol. 2015:1–9.
Christoph K, Raghu K. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl). 2013;91(4):431–7.
Li C, Liu DR, Li GG, Wang HH, Li XW, Zhang W, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroenterol. 2015;21(20):6215–28.
Yang TT, Zhang X, Wang M, Zhang J, Huang F, Cai J, et al. Activation of mesenchymal stem cells by macrophages prompts human gastric cancer growth through NF-κB pathway. PLoS ONE. 2014;9(5):e97569.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Li YS, Zhao LM, Shi BH, Ma SS, Xu Z, Ge Y, et al. Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Sci Rep. 2015;5:18648–63.
Cho HJ, Jung JI, Lim DY, Kwon GT, Her S, Park JH, et al. Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic model. Breast Cancer Res. 2012;14(3):R81.
Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–22.
Vella LJ. The emerging role of exosomes in epithelial mesenchymal transition in cancer. Front Oncol. 2014;4:361–6.
Fan GC. Hypoxic exosomes promote angiogenesis. Blood. 2014;124(25):3669–70.
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14(15):2473–83.
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–94.
Yu D, Wu Y, Zhang X, Lv M, Chen X, Yang SJ, et al. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumour Biol. 2015:1–9.
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4.
Gu JM, Qian H, Shen L, Zhang X, Zhu W, Huang L, et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS ONE. 2012;7(12):e52465.
Challagundla KB, Wise PM, Neviani P, Ghava H, Murtadha M, Kennedy R, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst. 2015;107(7):djv135.
Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–17.
Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6(30):29877–88.
Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–7.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. 2012;4(5):341–3.
Ma SS, Liu M, Xu ZB, Li YS, Guo H, Ge Y, et al. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget. 2016;7(12):13502–19.
Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750–66.
Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, et al. NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med. 2015;212(10):1725–38.
Yang Z, Xian H, Hu J, Tian S, Qin Y, Wang RF, et al. USP18 negatively regulates NF-κB signaling by targeting TAK1 and NEMO for deubiquitination through distinct mechanisms. Sci Rep. 2015;5:12738.
Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirlic C. Strazzabosco. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015;62(5):1551–62.
Bovy N, Blomme B, Frères P, Dederen S, Nivelles O, Lion M, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6(12):10253–66.
Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–97.
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–4.
Burke M, Choksawangkarn W, Edwards N, Ostrand-Rosenberg S, Fenselau C. Exosomes from myeloid-derived suppressor cells carry biologically active proteins. J Proteome Res. 2014;13(2):836–43.
Luga V, Zhang L, Viloria-Petit AM, Oqunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56.
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91.
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–6.
Kogure T, Yan IK, Lin WL, Patel T. Extracellular vesicle–mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261–72.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81572075, 81201660), the Natural Science Foundation of the Jiangsu Province (BK20141303), Jiangsu Province for Outstanding Sci-tech Innovation Team in Colleges and Universities (SJK2013-10), Jiangsu Province’s Outstanding Medical Academic Leader and Sci-tech Innovation Team Program (LJ201117), Jiangsu Province’s Major Project in Research and Development (BE2015667), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The medical ethics committee of Jiangsu University approved this study.
Conflicts of interest
None
Additional information
Lijun Wu and Xu Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Cell number counting of SGC-7901(a) and MGC-803(b) cells that were treated with the supernatant from cancer exosomes-activated macrophages for 5 days. ***P < 0.001; **P < 0.01; ns, no significance. (GIF 21 kb)
Fig. S2
Flow cytometry analyses of the apoptosis of SGC-7901(a) and MGC-803(b) cells after treatment with the supernatant from cancer exosomes-activated THP-1 cells for 24 h. (GIF 2393 kb)
Fig. S3
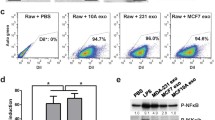
Western blot analyses of p-P65 and p65 protein levels in THP-1 cells at different time point after the treatment with GES-1 cells derived exosomes. (GIF 486 kb)
Fig. S4
Quantitative RT-PCR for IL-1β and IL-8 mRNA expression in primary macrophages pre-treated with 10 μM Bay 11–7082 for 2 h followed by stimulation with MGC-803 cells derived exosomes for 12 h. ***P < 0.001; **P < 0.01. (GIF 594 kb)
Rights and permissions
About this article
Cite this article
Wu, L., Zhang, X., Zhang, B. et al. Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumor Biol. 37, 12169–12180 (2016). https://doi.org/10.1007/s13277-016-5071-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5071-5