Abstract
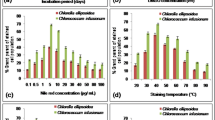
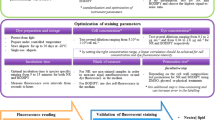
A simple and high-throughput method for determining in situ intracellular neutral lipid accumulation in Chlorella ellipsoidea and Chlorococcum infusionum with flow cytometry and confocal microscopy was established by employing different solvents and a lipophilic dye, Nile red. Seven different organic solvents, acetic acid, dimethyl sulfoxide (DMSO), acetone, methanol, ethanol, n-hexane, and chloroform at different concentrations ranging from 0 to 80% (v/v) were tested. The fluorescence signal for neutral lipids was collected with a 586/42 emission filter (PE-A) and the maximum fluorescence intensity (% grandparent) was measured as 74.01 ± 4.82% for Chlorella and 70.1 ± 5.52% for Chlorococcum at 30% acetic acid (v/v). The statistical analysis of Nile red-stained cells showed a high coefficient of variation (CV), standard deviation (SD), mean, and median values in the acetic acid-based staining method, followed by DMSO, n-hexane and chloroform. Confocal microscopy revealed a high rate of accumulation of cytosolic neutral lipids when stained with Nile red and other organic solvents. Higher lipid accumulation in Fesupplemented conditions was also detected and a maximum lipid content of 57.36 ± 0.41% (4-fold) in Chlorella and 48.20 ± 0.43% (4-fold) in Chlorococcum were measured at 0.001 g/L of ferrous sulfate (FeSO4). High fluorescence intensity (75.16 ± 0.24% in Chlorella and 72.24 ± 1.07% in Chlorococcum) in Fe-treated cells confirmed the efficiency of the staining procedure.
Similar content being viewed by others
References
Hu, Q., M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert, and A. Darzins (2008) Microalgal triacylglycerols as feed stocks for biofuel production: Perspective and advances. Plant J. 54: 621–639.
Kaewkannetra, P., P. Enmak, and T. Y. Chiu (2012) The Effect of CO2 and salinity on the cultivation of Scenedesmus obliquus for biodiesel production. Biotechnol. Bioproc. Eng. 17: 591–597.
Borowitzka, M. A. and N. R. Moheimani (2013) Sustainable biofuels from algae. Mitig. Adapt. Strategies Glob. Chang. 18: 13–25.
Khan, S., A. Rashmi, M. Z. Hussain, S. Prasad, and U. C. Banerjee (2009) Prospects of biodiesel production from microalgae in India. Renew. Sust. Energ. Rev. 13: 2361–2372.
Abou-Shanab, R. A. I., M. M. El-Dalatony, M. M. El-Sheekh, M. -K. Ji, E. -S. Salma, A. N. Kabra, and B. -H. Jeon (2014) Cultivation of a new microalga, Micractinium reisseri, in municipal wastewater for nutrient removal, biomass, lipid, and fatty acid production. Biotechnol. Bioproc. Eng. 19: 510–518.
Zhang, K., B. Sun, X. She, F. Zhao, Y. Cao, D. Ren, and J. Lu (2014) Lipid production and composition of fatty acids in Chlorella vulgaris cultured using different methods: Photoautotrophic, heterotrophic, and pure and mixed conditions. Ann. Microbiol. 64: 1239–1246.
Benemann, J. R. (2000) Hydrogen production by microalgae. J. Appl. Phycol. 12: 291–300.
Schenk, P. M., R. Skye, T. Hall, E. Stephens, U. C. Marx, J. H. Mussgnug, C. Posten, O. Kruse, and B. Hankamer (2008) Second generation biofuels: High efficiency microalgae for biodiesel production. Bioenerg. Res. 1: 20–43.
Battah, M., Y. El-Ayoty, A. E. -F. Abomohra, S. A. El-Ghany, and A. Esmael (2015) Effect of Mn2+, Co2+ and H2O2 on biomass and lipids of the green microalga Chlorella vulgaris as a potential candidate for biodiesel production. Ann. Microbiol. 65: 155–162.
Rashid, U., F. Anwar, B. R. Moser, and G. Knothe (2008) Moringa oleifera oil: A possible source of biodiesel. Bioresour. Technol. 99: 8175–8179.
Sharma, K. K., H. Schuhmann, and P. M. Schenk (2012) High lipid induction in microalgae for biodiesel production. Energie. 5: 1532–1553.
Huang, L., J. Xu, T. Li, L. Wang, T. Deng, and X. Yu (2014) Effects of additional Mg2+ on the growth, lipid production, and fatty acid composition of Monoraphidium sp. FXY-10 under different culture conditions. Ann. Microbiol. 64: 1247–1256.
Bligh, E. and W. J. Dyer (1959) A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917.
Engler, C. R. (1985) Disruption of microbial cells. pp. 305–324. 2nd ed., In: Moo-Yoong. M. (ed.). Comprehensive biotechnology. Pergamon Press, Oxford, UK.
Lee, S. J., B. D. Yoon, and H. M. Oh (1998) Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech. 12: 553–556.
Geciova, J., D. Bury, and P. Jelen (2002) Methods for disruption of microbial cells for potential use in the dairy industry-a review. Int. Dairy J. 12: 541–553.
Cravotto, G., L. Boffa, S. Mantegna, P. Ferego, M. Avogadro, and P. Cimas (2008) Improved extraction of vegetable oils under high-intensity ultrasound and /or microwaves. Ultrason. Sonochem. 15: 898–902.
Virot, M., V. Tomao, C. Ginies, F. Visinoni, and F. Chemat (2008) Microwave-integrated extraction of total fats and oils. J. Chromatogr. A. (1196-1197): 57–64.
Lee, J., C. Yoo, S. Jun, C. Ahn, and H. Oh (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 101: 575–577.
Chen, W., C. Zhang, L. Song, M. Sommerfeld, and Q. Hu (2009) A high throughput Nile Red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Meth. 77: 41–47.
Huang, G. H., G. Chen, and F. Chen (2009) Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass Bioenerg. 33: 1386–1392.
Katsuragi, T, and Y. Tani (2000) Screening for microorganisms with specific characteristics by flow cytometry and single-cell sorting. J. Biosci. Bioeng. 89: 217–222.
de la Jara, A., H. Mendoza, A. Martel, C. Molina, L. de la Nordstron, V. Rosa, R. Diaz (2003) Flow cytometric determination of lipid content in a marine dinoflagellate Crypthecodinium cohnii. J. Appl. Phycol. 15: 433–438.
Binet, M. T. and J. L. Stauber (2006) Rapid flow cytometric method for the assessment of toxic dinoflagellate cyst viability. Mar. Env. Res. 62: 247–260.
Guzman, H. M., A. de la Jara Valido, L. C. Duarte, and K. F. Presmanes (2010) Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture culture conditions. Aquacult. Int. 18: 189–199.
Pereira, H., L. Barreira, A. Mozes, C. Mlorindo, C. Polo, C. V. Duarte, L. Custodio, and J. Varela (2011) Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 4:61.
Yao, S., A. Brandt, E. Helge, and C. Gjermansen (2012) Neutral lipid accumulation at elevated temperature in conditional mutants of two microalgae species. Plant Physiol. Biochem. 61: 71–79.
Doan, T. T. H. and J. P. Obbard (2012) Enhanced intracellular lipid in Nannochloropsis sp. via random mutagenesis and flow cytometric cell sorting. Algal Res. 1: 17–21.
Roleda, M. Y., S. P. Slocombe, R. J. G. Leakey, J. G. Day, E. M. Bell, and M. S. Stanley (2013) Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour. Technol. 129: 439–449.
Hyka, P., S. Lickova, P. Pribyl, K. Melzoch, and K. Kovar (2013) Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol. Adv. 31: 2–16.
Velmurugan, N., M. Sung, S. S. Yim, M. S. Park, J. W. Yang, and K. J. Jeong (2013) Evaluation of intracellular lipid bodies in Chlamydomonas reinhardtii strains by flow cytometry. Bioresour. Technol. 138: 30–37.
Satpati, G. G. and R. Pal (2015) Rapid detection of neutral lipid in green microalgae by flow cytometry in combination with Nile red staining-an improved technique. Ann. Microbiol. 65: 937–949.
Fakhry, E. M. and D. M. El Maghraby (2015) Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Bot. Stud. 56:6.
Li, C., Y. Yu, D. Zhang, J. Liu, N. Ren, and Y. Feng (2014) Combined effects of carbon, phosphorus and nitrogen on lipid accumulation of Chlorella vulgaris in mixotrophic culture. J. Chem. Technol. Biotechnol. DOI 10.1002/jctb.4623.
Shah, S. M. U., C. C. Radziah, S. Ibrahim, F. Latiff, M. F. Othman, and M. A. Abdullah (2014) Effects of photoperiod, salinity and pH on cell growth and lipid content of Pavlova lutheri. Ann. Microbiol. 64: 157–164.
Sasireka, G. and R. Muthuvelayudham (2015) Effect of salinity and iron stressed on growth and lipid accumulation In Skeletonema costatum for biodiesel production. Res. J. Chem. Sci. 5: 69–72.
Liu, Z. -Y., G. -C. Wang, and B. -C. Zhou (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 99: 4717–4722.
Deng, X., X. Fei, and Y. Li (2011) The effects of nutritional restriction on neutral lipid accumulation in Chlamydomonas and Chlorella. African J. Microbiol. Res. 5: 260–270.
Praveenkumar, R., K. Shameera, G. Mahalakshmi, M. A. Akbarsha, and N. Thajuddin (2012) Influence of nutrient deprivation on lipid accumulation in a dominant indigenous microalga Chlorella sp., bum11008: Evaluation for biodiesel production. Biomass Bioenerg. 37: 60–66.
Ruangsomboon, S. (2012) Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresour. Technol. 109: 261–265.
Concas, A., A. Steriti, M. Pisu, and G. Cao (2014) Comprehensive modeling and investigation of the effect of iron on the growth rate and lipid accumulation of Chlorella vulgaris cultured in batch photobioreactors. Bioresour. Technol. 153: 340–350.
Zarrouk, C. (1966) Contribution a letude dune cyanophycee. Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima. Ph.D Thesis, Universite de Paris.
Bold, H. C. (1949) The morphology of Chlamydomonas chlamydogama sp. nov. Bull. Torrey. Bot. Club. 76: 101–108.
Dou, X., X. H. Lu, M. Z. Lu, L. S. Yu, R. Xue, and J. B. Ji (2013) The effects of trace elements on the lipid productivity and fatty acid composition of Nannochloropsis oculata. J. Renew. Energ. DOI: 10.1155/2013/671545.
Hu, Q. (2004) Environmental effects on cell composition. pp. 83–93. In: Richmond, A. (ed.). Handbook of Microalgal Culture: Biotechnology and applied phycology. Blackwell Science, Victoria, Australia.
Zhiyuan, L. and W. Guangce (2014) Effect of Fe3+ on the growth and lipid content of Isochrysis galbana. Chinese J. Oceanol. Limnol. 32: 47–53.
Kean, M. A., E. Brons Delgado, B. P. Mensink, and M. H. J. Bugter (2015) Iron chelating agents and their effects on the growth of Pseudokirchneriella subcapitata, Chlorella vulgaris, Phaeodactylum tricornutum and Spirulina platensis in comparison to Fe-EDTA. J. Algal Biomass Utln. 6: 56–73.
Seenuvasan, M., K. S. Kumar, S. Abhinandan, C. Anugraha, K. Umamageshwari, M. A. Kumar, and N. Balaji (2014) Statistical analysis on stress induced lipid accumulation along with the major cell components of Chlorella sp. Int. J. Chem. Tech. Res. 6: 4186–4192.
Seenuvasan, M., P. K. Selvi, M. A. Kumar, J. Iyyappan, and K. S. Kumar (2014) Standardization of non-edible Pongamia pinnata oil methyl ester conversion using hydroxyl content and GC-MS analysis. J. Taiwan Inst. Chem. Eng. 45: 1485–1489.
Cirulis, J. T., B. C. Strasser, J. A. Scott, and G. M. Ross (2012) Optimization of staining conditions for microalgae with three lipophilic dyes to reduce precipitation and fluorescence variability. Cytometry Part A 81A: 618–626.
Cooksey, K. E., J. B. Guckert, S. Williams, and P. R. Callis (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J. Microbiol. Method. 6: 333–345.
Doan, T. T. Y. and J. P. Obbard (2011) Improved Nile Red staining of Nannochloropsis sp. J. Appl. Phycol. 23: 895–901.
Guzman, H. M., A. de la Jara Valido, L. C. Duarte, and K.F. Presmanes (2011) Analysis of Intraspecific variation in relative fatty acid composition: Use of flow cytometry to estimate unsaturation index and relative polyunsaturated fatty acid content in microalgae. J. Appl. Phycol. 23: 7–15.
Fuentes-Grunewald, C., E. Garces, E. Alacid, N. Sampedro, S. Rossi, and J. Camp (2012) Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J. Ind. Microbiol. Biotechnol. 39: 207–216.
Lopes da Silva, T., A. Reis, R. Medeiros, A. C. Oliveira, and L. Gouveia (2009) Oil production towards biofuel from autotrophic microalgae: a semicontinuous cultivations monitorized by flow cytometry. Appl. Biochem. Biotechnol. 159: 568–578.
Davey, H. M. and D. B. Kell (1996) Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol. Rev. 60: 641–696.
Wang, Y., F. Hammes, K. De Roy, W. Verstraete, and N. Boon (2010) Past, present and future applications of flow cytometry in aquatic microbiology. Trends Biotechnol. 28: 416–424.
Fuentes-Grunewald, C., E. Garces, S. Rossi, and J. Camp (2009) Use of dinoflagellate Karlodinium veneficum as a sustainable source of biodiesel production. J. Ind. Microbiol. Biotechnol. 36: 1215–1224.
Farese, R. V. and T. C. Walther (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cel. 139: 855–860.
Xu, D., Z. Gao, F. Li, X. Fan, X. Zhang, N. Ye, S. Mou, C. Liang, and D. Li (2013) Detection and quantification of lipid in the microalga Tetraselmis subcordiformis (Wille) Butcher with BODIPY505/515 staining. Bioresour. Technol. 127: 386–390.
Brennan, L., A. B. Fernandez, A. S. Mostaert, and P. Owende (2012) Enhancement of BODIPY505/515 lipid fluorescence method for applications in biofuel-directed microalgae production. J. Microbiol. Method. 90: 137–143.
Yeesang, C. and B. Cheirsilp (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 102: 3034–3040.
Fan, J., Y. Cui, M. Wan, W. Wang, and Y. Li (2014) Lipid accumulation and biosynthesis genes response of the Oleaginous Chlorella pyrenoidosa under three nutritional stressors. Biotechnol. Biofuels 7: 17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satpati, G.G., Mallick, S.K. & Pal, R. An alternative high-throughput staining method for detection of neutral lipids in green microalgae for biodiesel applications. Biotechnol Bioproc E 20, 1044–1055 (2015). https://doi.org/10.1007/s12257-015-0281-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-015-0281-z