Abstract
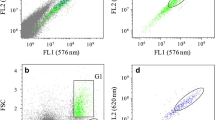
The present work shows the possibility of determining variations in the lipid composition in Tetraselmis suecica under different conditions of culture by means of flow cytometry in cells marked with Nile Red (NR). A significant correlation was observed between the cellular contents in polar and neutral lipids and the cytometric signal of the marked cells. Likewise, there was a significant correlation between the ratio of polar and neutral lipids, estimated by cytometry, and the relative composition of polyunsaturated fatty acids (PUFAs) in Tetraselmis, which corresponded to the greater content of PUFAs detected in the polar lipid fraction of this microalgae. This relationship between the polar/neutral ratio and the relative contents of PUFAs, together with the flow cytometry and the marking by means of NR, would make it possible to have an effective indicator of the abundance of PUFAs in Tetraselmis, as well as the development of techniques of massive screening of strains which are hyperproductive of PUFAs and of rapid checking of the variations in lipid composition in response to cultivation conditions, which are much simpler and more rapid than traditional techniques.




Similar content being viewed by others
Abbreviations
- PUFAs:
-
Polyunsaturated fatty acids
- NR:
-
Nile Red
- SATs:
-
Saturated fatty acids
- MUFAs:
-
Monounsaturated fatty acids
References
Alonzo F, Mayzaud P (1999) Spectrofluorometric quantification of neutral and polar lipids in zooplankton using Nile Red. Mar Chem 67:289–301. doi:10.1016/S0304-4203(99)00075-4
Bonaldo A, Badiani A, Testi S, Corso G, Mordenti AL, Gatta PP (2005) Use of centrifuged and preserved microalgae for feeding juvenile Manila clam (Tapes philippinarum): effects on growth and fatty acid composition. Ital J Anim Sci 4:375–384
Cooksey KE, Guckert JB, Williams GA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6:333–346. doi:10.1016/0167-7012(87)90019-4
D’Souza FML, Kelly GJ (2000) Effects of a diet of a nitrogen-limited alga (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture 181:311–329. doi:10.1016/S0044-8486(99)00231-8
Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations—the importance of single-cell analyses. Microbiol Rev 60:641–696
De la Jara A, Mendoza H, Martel A, Molina C, Nordströn C, de la Rosa V, Díaz R (2003) Flow cytometric determination of lipid content in marine dinoflagellate Crypthecodinium cohnii. J Appl Phycol 15:433–438. doi:10.1023/A:1026007902078
De la Pena MR, Villegas CT (2005) Cell growth, effect of filtrate and nutritive value of the tropical Prasinophyte Tetraselmis tetrathele (Butcher) at different phases of culture. Aquacult Res 36:1500–1508. doi:10.1111/j.1365-2109.2005.01371.x
El-Dakar AY, Shalaby SM, Hassanein GD, Ghoneim SI (2001) Use of rotifers cultured on different microalgal species in larval feeding of sea bass Dicentrarchus labrax. Asian Fish Sci 14:43–52
Fábregas J, Otero A, Domínguez A, Patino M (2001) Growth rate of the microalga Tetraselmis suecica changes the biochemical composition of Artemia species. Marin Biotechnol 3:256–263. doi:10.1007/s101260000074
Floreto EAT, Hirata H, Yamasaki S, Castro SC (1994) Influence of light intensity on the fatty acid composition of Ulva pertuosa kjellman (Chlorophyta). Bot Mar 37:143–149
Guillard RRL (1975) Culture of phytoplakton for feeding marine invertebrates. In: Smith WL, Chanle MH (eds) Culture invertebrate animals. Plenum, New York, pp 26–60
Guimarães ARP, Costa Rosa LFBP, Sitnik RH, Curi R (1991) Effect of polyunsaturaes (PUFA n-6) and saturated fatty acid-rich diets on macrophage metabolism and function. Biochem Int 23:1739–1751
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Darzins A (2008) Micoalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. doi:10.1111/j.1365-313X.2008.03492.x
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids in rat heart using silica cartridges. Lipids 20:40–41. doi:10.1007/BF02534360
Kimura K, Yamaoka M, Kamisaka Y (2004) Rapid estimation of lipids in oleaginous fungi and yeast using Nile Red fluorescence. J Microbiol Methods 56:331–338. doi:10.1016/j.mimet.2003.10.018
Lavens P, Sorgeloos P (1996) Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper no. 361, Roma, 295 pp
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722. doi:10.1016/j.biortech.2007.09.073
Mendoza H, Martel A, Jiménez del Río M, García-Reina G (1999) Oleic acid is the main fatty acid related with carotenogenesis in Dunaliella salina. J Appl Phycol 11:15–19. doi:10.1023/A:1008014332067
Mendoza H, Molina Cedrés C, de la Jara A, Nordström L, Freijanes K, Carmona L (2008) Quantitative and qualitative variation of the fatty acid composition in the dinoflagellate Cryothecodinium cohnii under nitrogen starvation conditions. Grasas Aceites 59:27–32. doi:10.3989/gya.2008.v59.i1.486
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12:527–534. doi:10.1023/A:1008106304417
Otero A, Fábregas J (1997) Changes in the nutrient composition of Tetraselmis suecica cultured semicontinuously with different nutrient concentrations and renewal rates. Aquaculture 159:111–123. doi:10.1016/S0044-8486(97)00214-7
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 15:1–9. doi:10.1007/s10499-006-9060-3
Petkov G, García G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35:281–285. doi:10.1016/j.bse.2006.10.017
Pratoomyot J, Srivilas P, Noiraksar T (2005) Fatty acids composition of 10 microalgal species. Songklanakarin J Sci Technol 27:1179–1187
Pulz O, Cross W (2004) Valuable products from biotechnology of microalgae. J Appl Microbiol Biot 65:635–648. doi:10.1007/s00253-004-1647-x
Radner RJ, Parker BC (1994) Commercial applications of algae: opportunities and constraints. J Appl Phycol 6:93–98. doi:10.1007/BF02186062
Roessler PG (1990) Environmental control of glycerolipids metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399. doi:10.1111/j.0022-3646.1990.00393.x
Rosa A, Deidda D, Serra A, Deiana M, Dessì MA, Pompei R (2005) Omega-3 fatty acid composition and biological activity of three microalgae species. J Food Agric Environ 3:120–124
Sukenik A, Carmeli Y (1990) Lipid synthesis and fatty acid composition in nannochloropsis sp (Eustigmatophyceae) grown in light-dark cycle. J Phycol 26:463–469. doi:10.1111/j.0022-3646.1990.00463.x
Swaaf ME (2003) Docohexaenoic acid production by the marine alga Crypthecodinium cohnii. University Press, Netherlands, p 125
Thomson PA, Harrison PJ, Whyte JNC (1990) Influence of irradiance on the fatty acid composition of phytoplankton. J Phycol 26:278–288. doi:10.1111/j.0022-3646.1990.00278.x
Tzovenis I, De Pauw N, Sorgeloos P (2003) Optimisation of T-ISO biomass production rich in essential fatty acids II. Effect of different light regimes on the production of fatty acids. Aquaculture 216:223–242. doi:10.1016/S0044-8486(02)00375-7
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of productions. Process Biochem 40:3267–3652. doi:10.1016/j.procbio.2005.02.020
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docohexaenoic acid production by Schizochytrium limacinum SR21. Appl Microbiol Biotechnol 49:72–76. doi:10.1007/s002530051139
Yongmanitchai W, Ward OP (1992) Separation of lipid classes from Phaeodactylum tricornutum using silica cartridges. Phytochemistry 31:3405–3408. doi:10.1016/0031-9422(92)83694-T
Acknowledgment
This work was financed by the program “Canarias Objetivo de Progreso” (2007-2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzmán, H.M., de la Jara Valido, A., Duarte, L.C. et al. Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult Int 18, 189–199 (2010). https://doi.org/10.1007/s10499-008-9235-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-008-9235-1