Abstract
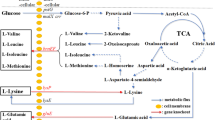
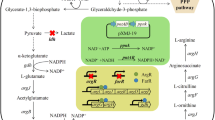
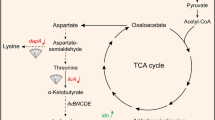
Our previous work has shown that L-isoleucine production in Corynebacterium glutamicum IWJ001 could be increased by overexpressing ilvA1 encoding a feedback-resistant threonine dehydratase, ilvBN1 encoding a feedback-resistant acetohydroxy acid synthase, lrp encoding the global regulator Lrp, brnFE encoding the two-component export system BrnFE, or ppnk1 encoding NAD kinase. The main purpose of this study is to further increase the L-isoleucine production in C. glutamicum IWJ001 by overexpressing the above genes in various combinations. Several C. glutamicum strains IWJ001/pDXW-8-ppnk1-lrp-brnFE, IWJ001/pDXW-8-ilvBN1-ilvA1-lrp-brnFE, IWJ001/pDXW-8-ilvBN1-ilvA1-ppnk1, and IWJ001/pDXW-8-ppnk1-ilvBN1-ilvA1-lrp-brnFE were constructed, and L-isoleucine production and activities of several key enzymes in these strains were analyzed. Compared with the control strain IWJ001/pDXW-8, L-isoleucine production increased in all of the four strains. IWJ001/pDXW-8-ilvBN1-ilvA1-ppnk1 showed the highest L-isoleucine production and produced 32.3 g/L L-isoleucine in 72 h fed batch fermentation. The results indicate that L-isoleucine production in C. glutamicum could be increased by enhancing the carbon flux and NADPH supply in the biosynthetic pathway.
Similar content being viewed by others
References
Park, J. H. and S. Y. Lee (2010) Metabolic pathways and fermentative production of L-aspartate family amino acids. J. Biotechnol. 5: 560–577.
Eggeling, L., S. Morbach, and H. Sahm (1997) The fruits of molecular physiology: Engineering the L-isoleusine biosynthesis pathway in Corynebacterium glutamicum. J. Biotechnol. 56: 167–182.
Umbarger, H. E. (1987) Biosynthesis of the branched-chain amino acids. pp. 352–367. In: F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (eds.). Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington D. C., USA.
Morinaga, Y., H. Takagi, M. Ishida, K. Miwa, T. Sato, S. Nakamori, and K. Sano (1987) Threonine production by co-existence of cloned genes coding homoserine dehydrogenase and homoserine kinase in Brevibacterium lactofermentum. Agric. Biol. Chem. 51: 93–100.
Colon, G. E., M. S. M. Jetten, T. T. Nguyen, M. E. Gubler, M. T. Follettie, A. J. Sinskey, and G. Stephanopoulos (1994) Effect of inducible thrB expression on amino acid production in Corynebacterium lactofermentum ATCC 21799. Appl. Environ. Microbiol. 61: 74–78.
Dong, X., P. J. Quinn, and X. Wang (2011) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of L-threonine. Biotechnol. Adv. 29: 11–23.
Morbach, S., H. Sahm, and L. Eggeling (1995) Use of feedbackresistant threonine dehydratases of Corynebacterium glutamicum to increase carbon flux towards L-isoleucine. Appl. Environ. Microbiol. 61: 4315–4320.
Elišáková, V., M. Pátek, J. Holátko, J. Nešvera, D. Leyval, J. L. Goergen, and S. Delaunay (2005) Feedback-resistant acetohydroxy acid synthase increases valine production in Corynebacterium glutamicum. Appl. Environ. Microbiol. 71: 207–213.
Kennerknecht, N., H. Sahm, M.-R. Yen, M. Pátek, M. H. Saier, and L. Eggeling (2002) Export of L-isoleucine from Corynebacterium glutamicum: A two-gene-encoded member of a new translocator family. J. Bacteriol. 184: 3947–3956.
Yin, L., F. Shi, X. Hu, C. Chen, and X. Wang (2013) Increasing L-isoleucine production in Corynebacterium glutamicum by overexpressing global regulator Lrp and two-component export system BrnFE. J. Appl. Microbiol. 114: 1369–1377.
Ikeda, S., I. Fujita, and F. Yoshinaga (1976) Screening of L-isoleucine producers among ethionine resistant mutants of L-threonine producing bacteria. Agric. Biol. Chem. 40: 511–516.
Kase, H. and K. Nakayama (1977) L-Isoleucine production by analog-resistant mutants derived from threonine-producing strain of Corynebacterium glutamicum. Agric. Biol. Chem. 41: 109–116.
Kalinowski, J., B. Bathe, D. Bartels, N. Bischoff, M. Bott, A. Burkovski, N. Dusch, L. Eggeling, B. J. Eikmanns, and L. Gaigalat (2003) The complete Corynebacterium glutamicum ATCC13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 104: 5–25.
Morbach, S., R. Kelle, S. Winkels, H. Sahm, and L. Eggeling (1996) Engineering the homoserine dehydrogenase and threonine dehydratase control points to analyse flux towards L-isoleucine in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 45: 612–620.
Eggeling, L., H. Sahm, and A. A. de Graaf (1996) Quantifying and directing metabolite flux: Application to amino acid overproduction. Metab. Eng. 54: 1–30.
Yin, L., X. Hu, D. Xu, J. Ning, J. Chen, and X. Wang (2012) Coexpression of feedback-resistant threonine dehydratase and acetohydeoxy acid synthase increase L-isoleucine production in Corynebacterium glutamicum. Metab. Eng. 14: 542–550.
Shi, F., X. J. Huan, X. Y. Wang, and J. F. Ning (2012) Overexpression of NAD kinases improves the L-isoleucine biosyntheis in Corynebacterium glutamicum ssp. lactofermentum. Enz. Microb. Tech. 51: 73–80.
Peng, Z. J., J. Fang, J. H. Li, L. Liu, G. C. Du, J. Chen, X. Y. Wang, J. F. Ning, and L. M. Cai (2010) Combined dissolved oxygen and pH control strategy to improve the fermentative production of L-isoleucine by Brevibacterium lactofermentum. Bioproc. Biosyst. Eng. 33: 339–345.
Sambrook, J. and R. W. Russel (2001) Molecular cloning: A laboratory manual. 3rd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA.
Xu, D., Y. Tan, X. Huan, X. Hu, and X. Wang (2010) Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. J. Microbiol. Meth. 80: 86–92.
Miller, L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 31: 426–428.
Koros, A., Z. S. Varga, and P. Molnar (2008) Simultaneous analysis of amino acids and amines as their ophthalaldehydeethanethiol-9-fluorenylmethyl chloroformate derivatives in cheese by high-performance liquid chromatography. J. Chromatogr. A. 1203: 146–152.
Livak, K. J. and T. D. Schmittgen (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC T method. Methods 25: 402–408.
Nolden, L., M. Farwick, R. Krämer, and Burkovski (2001) Glutamine synthetases of Corynebacterium glutamicum: Transcriptional control and regulation of activity. FEMS Microbiol. Lett. 201: 91–98.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Miyajima, R., S. -I. Otsuka, and I. Shiio (1968) Regulation aspartate family amino acid biosynthesis in Brevibacterium flavum. J. Biochem. 63: 139–148.
Guillouet, S., A. A. Rodal, and A. J. Sinskey (1999) Expression of the Escherichia coli catabolic threonine dehydratase in Corynebacterium glutamicum and its effect on isoleucine production. Appl. Environ. Microbiol. 65: 3100–3107.
Westerfeld, W. W. (1945) A colorimetrie determination of blood acetoin. J. Biol. Chem. 161: 495–502.
Eggeling, I., L. Eggeling, and H. Sahm (1987) Regulation of acetohydroxy acid synthase in Corynebacterium glutamicum during fermentation of α-ketobutyrate to L-isoleucine. Appl. Microbiol. Biotechnol. 25: 346–351.
Shi, F., S. Kawai, S. Mori, E. Kono, and K. Murata (2005) Identification of ATP-NADH kinase isozymes and their contribution to supply of NADP(H) in Saccharomyces cerevisiae. FEBS. J. 272: 3337–3379.
Lee, I. Y., M. K. Kim, Y. H. Park, and S. Y. Lee (1996) Regulatory effects of cellular nicotinamide nucleotides and enzyme activities on poly(3-hydroxybutyrate) synthesis in recombinant Escherichia coli. Biotechnol. Bioeng. 52: 707–712.
Zerez, C. R., S. J. Lee, and K. R. Tanaka (1987) Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal. Biochem. 164: 367–373.
Kawa, I. S., S. Mori, T. Mukai, W. Hashimoto, and K. Murata (2001) Molecular characterization of Escherichia coli NAD kinase. Eur. J. Biochem. 268: 4359–4365.
Grose, J. H., L. Joss, S. F. Velick, and J. R. Roth (2006) Evidence that feedback inhibition of NAD kinase controls responses to oxidative stress. Proc. Natl. Acad. Sci. USA 103: 7601–7606.
Sauer, U., F. Canonaco, S. Heri, A. Perrenoud, and E. Fischer (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J. Biol. Chem. 279: 6613–6619.
Koffas, M. and G. Stephanopoulos (2005) Strain improvement by metabolic engineering: lysine production as a case study for systems biology. Curr. Opin. Biotechnol. 16: 361–366.
Lee, K. H., J. H. Park, T. Y. Kim, H. U. Kim, and S. Y. Lee (2007) Systems metabolic engineering of Escherichia coli for L-threonine production. Mol. Syst. Biol. 3: 149.
Kind, S., J. Becher, and C. Wittmann (2012) Increased lysine production by flux coupling of the tricarboxylic acid cycle and the lysine biosynthetic pathway-Metabolic engineering of the availability of succinyl-CoA in Corynebacterium glutamicum. Metab. Eng. 15: 184–195.
Becker, J., O. Zelder, S. Häfner, H. Schröder, and C. Wittmann (2011) From zero to hero-Design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production. Metab. Eng. 13: 159–168.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, L., Zhao, J., Chen, C. et al. Enhancing the carbon flux and NADPH supply to increase L-isoleucine production in Corynebacterium glutamicum . Biotechnol Bioproc E 19, 132–142 (2014). https://doi.org/10.1007/s12257-013-0416-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-013-0416-z