Abstract
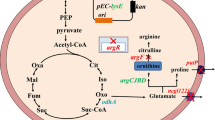
Corynebacterium glutamicum SNK 118 was metabolically engineered with improved l-arginine titer. Considering the crucial role of NADPH level in l-arginine production, pntAB (membrane-bound transhydrogenase) and ppnK (NAD+ kinase) were co-expressed to increase the intracellular NADPH pool. Expression of pntAB exhibited significant effects on NADPH supply and l-arginine synthesis. Furthermore, argR and farR, encoding arginine repressor ArgR and transcriptional regulator FarR, respectively, were removed from the genome of C. glutamicum. The competitive branch pathway gene ldh was also deleted. Eventually, an engineered C. glutamicum JML07 was obtained for l-arginine production. Fed-batch fermentation in 5-L bioreactor employing strain JML07 allowed production of 67.01 g L−1l-arginine with productivity of 0.89 g L−1 h−1 and yield of 0.35 g g−1 glucose. This study provides a productive l-arginine fermentation strain and an effective cofactor manipulating strategy for promoting the biosynthesis of NADPH-dependent metabolites.





Similar content being viewed by others
References
Bartek T, Blombach B, Lang S, Eikmanns BJ, Wiechert W, Oldiges M, Nöh K, Noack S (2011) Comparative C-13 metabolic flux analysis of pyruvate dehydrogenase complex-deficient, l-valine-producing Corynebacterium glutamicum. Appl Environ Microbiol 77(18):6644–6652. https://doi.org/10.1128/AEM.00575-11
Blombach B, Riester T, Wieschalka S, Ziert C, Youn JW, Wendisch VF, Eikmanns BJ (2011) Corynebacterium glutamicum tailored for efficient isobutanol production. Appl Environ Microbiol 77(10):3300–3310. https://doi.org/10.1128/AEM.02972-10
Chemler JA, Fowler ZL, McHugh KP, Koffas MA (2010) Improving NADPH availability for natural product biosynthesis in Escherichia coli by metabolic engineering. Metab Eng 12:96–104. https://doi.org/10.1016/j.ymben.2009.07.003
Chen C, Li Y, Hu J, Dong X, Wang X (2015) Metabolic engineering of Corynebacterium glutamicum ATCC13869 for l-valine production. Metab Eng 29:66–75. https://doi.org/10.1016/j.ymben.2015.03.004
Cheng F, Luozhong S, Guo Z, Yu H, Stephanopoulos G (2017) Enhanced biosynthesis of hyaluronic acid using engineered Corynebacterium glutamicum via metabolic pathway regulation. J Biotechnol 12:1700191
Chen Z, Huang J, Wu Y, Wu W, Zhang Y, Liu D (2017) Metabolic engineering of Corynebacterium glutamicum for the production of 3-hydroxypropionic acid from glucose and xylose. Metab Eng 39:151–158. https://doi.org/10.1016/j.ymben.2016.11.009
Hänßler E, Müller T, Jeßberger N, Völzke A, Plassmeier J, Kalinowski J, Burkovski A (2007) FarR, a putative regulator of amino acid metabolism in Corynebacterium glutamicum. Appl Microbiol Biotechnol 76:625–632. https://doi.org/10.1007/s00253-007-0929-5
Hou X, Chen X, Zhang Y, Qian H, Zhang W (2012) Improvement of l-lysine production combines with minimization of by-products synthesis in Corynebacterium glutamicum. Amino Acids 43(6):2301–2311. https://doi.org/10.1007/s00726-012-1308-9
Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M (2009) Reengineering of a Corynebacterium glutamicum l-arginine and l-citrulline producer. Appl Environ Microbiol 75:1635–1641
Jackson JB (2003) Proton translocation by transhydrogenase. FEBS Lett 545:18–24
Jorge JM, Nguyen AQ, Perez-Garcia F, Kind S, Wendisch VF (2017) Improved fermentative production of gamma-aminobutyric acid via the putrescine route: systems metabolic engineering for production from glucose, amino sugars, and xylose. Biotechnol Bioeng 114:862–873
Kabus A, Georgi T, Wendisch VF, Bott M (2007) Expression of the Escherichia coli pntAB genes encoding a membrane-bound transhydrogenase in Corynebacterium glutamicum improves l-lysine formation. Appl Microbiol Biotechnol 75:47–53. https://doi.org/10.1007/s00253-006-0804-9
Kawai S, Mori S, Mukai T, Hashimoto W, Murata K (2001) Molecular characterization of Escherichia coli NAD kinase. FEBS J 268:4359–4365
Kim SY, Lee J, Lee SY (2015) Metabolic engineering of Corynebacterium glutamicum for the production of l-ornithine. Biotechnol Bioeng 112:416–421
Lee HC, Kim JS, Jang W, Kim SY (2010) High NADPH/NADP+ ratio improves thymidine production by a metabolically engineered Escherichia coli strain. J Biotechnol 149:24–32. https://doi.org/10.1016/j.jbiotec.2010.06.011
Lindner SN, Niederholtmeyer H, Schmitz K, Schoberth SM, Wendisch VF (2010) Polyphosphate/ATP-dependent NAD kinase of Corynebacterium glutamicum: biochemical properties and impact of ppnK overexpression on lysine production. Appl Microbiol Biotechnol 87:583–593. https://doi.org/10.1007/s00253-010-2481-y
Li ZJ, Cai L, Wu Q, Chen GQ (2009) Overexpression of NAD kinase in recombinant Escherichia coli harboring the phbCAB operon improves poly (3-hydroxybutyrate) production. Appl Microbiol Biotechnol 83:939–947. https://doi.org/10.1007/s00253-009-1943-6
Man Z, Rao Z, Xu M, Guo J, Yang T, Zhang X, Xu Z (2016) Improvement of the intracellular environment for enhancing l-arginine production of Corynebacterium glutamicum by inactivation of H2O2-forming flavin reductases and optimization of ATP supply. Metab Eng 38:310–321. https://doi.org/10.1016/j.ymben.2016.07.009
Man Z, Xu M, Rao Z, Guo J, Yang T, Zhang X, Xu Z (2016) Systems pathway engineering of Corynebacterium crenatum for improved l-arginine production. Sci Rep 6:28629
Meiswinkel TM, Rittmann D, Lindner SN, Wendisch VF (2013) Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum. Bioresour Technol 145:254–258. https://doi.org/10.1016/j.biortech.2013.02.053
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moritz B, Striegel K, de Graaf AA, Sahm H (2000) Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose phosphate pathway flux in vivo. FEBS J 267:3442–3452
Park SH, Kim HU, Kim TY, Park JS, Kim SS, Lee SY (2014) Metabolic engineering of Corynebacterium glutamicum for l-arginine production. Nat Commun 5:4618
Plassmeier J, Li Y, Rueckert C, SinJMLey AJ (2016) Metabolic engineering Corynebacterium glutamicum to produce triacylglycerols. Metab Eng 33:86–97. https://doi.org/10.1016/j.ymben.2015.11.002
Qiao K, Wasylenko TM, Zhou K, Xu P, Stephanopoulos G (2017) Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat Biotechnol 35:173–177
Sauer U, Canonaco F, Heri S, Perrenoud A, Fischer E (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279:6613–6619
Shi A, Zhu X, Lu J, Zhang X, Ma Y (2013) Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10. https://doi.org/10.1016/j.ymben.2012.11.008
Shi F, Huan X, Wang X, Ning J (2012) Overexpression of NAD kinases improves the l-isoleucine biosynthesis in Corynebacterium glutamicum ssp. lactofermentum. Enzyme Microb Tech 51:73–80. https://doi.org/10.1016/j.enzmictec.2012.04.003
Shin JH, Lee SY (2014) Metabolic engineering of microorganisms for the production of l-arginine and its derivatives. Microb Cell Fact 13:166
Siebert D, Wendisch VF (2015) Metabolic pathway engineering for production of 1, 2-propanediol and 1-propanol by Corynebacterium glutamicum. Biotechol Biofuels 8:91. https://doi.org/10.1186/s13068-015-0269-0
Takeno S, Hori K, Ohtani S, Mimura A, Mitsuhashi S, Ikeda M (2016) l-Lysine production independent of the oxidative pentose phosphate pathway by Corynebacterium glutamicum with the Streptococcus mutans gapN gene. Metab Eng 37:1–10. https://doi.org/10.1016/j.ymben.2016.03.007
Theron G, Reid SJ (2011) ArgR-promoter interactions in Corynebacterium glutamicum arginine biosynthesis. Appl Biochem Biothech 58:119–127
Van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52:541–545. https://doi.org/10.1007/s002530051557
Xu D, Tan Y, Huan X, Hu X, Wang X (2010) Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. J Microbiol Meth 80:86–92. https://doi.org/10.1016/j.mimet.2009.11.003
Yamauchi Y, Hirasawa T, Nishii M, Furusawa C, Shimizu H (2014) Enhanced acetic acid and succinic acid production under microaerobic conditions by Corynebacterium glutamicum harboring Escherichia coli transhydrogenase gene pntAB. J Gen Appl Microbiol 60:112–118
Yin L, Zhao J, Chen C, Hu X, Wang X (2014) Enhancing the carbon flux and NADPH supply to increase l-isoleucine production in Corynebacterium glutamicum. Biotechnol Bioproc E 19:132–142. https://doi.org/10.1007/s12257-013-0416-z
Wang C, Zhou Z, Cai H, Chen Z, Xu H (2017) Redirecting carbon flux through pgi-deficient and heterologous transhydrogenase toward efficient succinate production in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 44(7):1115–1126. https://doi.org/10.1007/s10295-017-1933-0
Wang Y, San KY, Bennett GN (2013) Cofactor engineering for advancing chemical biotechnology. Curr Opin Biotech 24:994–999. https://doi.org/10.1016/j.copbio.2013.03.022
Acknowledgements
This work was supported by National Natural Science Foundation of China (31601463), National First-class Discipline Program of Light Industry Technology and Engineering (LITE2018-07), Six Talent Peaks Project of Jiangsu Province (2015-SWYY-008), the Program of Introducing Talents of Discipline to Universities (111-2-06), and the Collaborative Innovation Center of Jiangsu Modern Industrial Fermentation. We are grateful to Prof. Xiaoyuan Wang (Jiangnan University, China) for kind assistance on construction of C. glutamicum strains.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhan, M., Kan, B., Dong, J. et al. Metabolic engineering of Corynebacterium glutamicum for improved l-arginine synthesis by enhancing NADPH supply. J Ind Microbiol Biotechnol 46, 45–54 (2019). https://doi.org/10.1007/s10295-018-2103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2103-8