Abstract
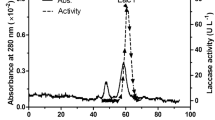
Production of laccase using a submerged culture of Trametes versicolor sdu-4 was optimized using a central composite design of the Response Surface Methodology. Optimized conditions gave a laccase yield of 4,213 U/L which was approximately three times of that in basal medium. The laccase was purified to homogeneity using a three-step process. The overall yield of the purification was 58%, with a purification fold of 11.4 and a specific activity of 1320.7 U/mg protein. The molecular mass of the laccase was 60 kDa. The optimum pH values of the enzyme were 2.2, 3.7, and 7 for the oxidations of ABTS, DMP, and syringaldazine, respectively. The enzyme had adaptability to a broad pH range and high temperature and wsa stable at pH 3.0 ∼ 10.0. The half-life of this laccase at 70°C was 2.2 h. Methyl red, 2-bromophenol, and 4-bromophenol were oxidized by the purified laccase in the absence of mediators. Purified laccase was effective in the decolorization of several dyes and was not inhibited by Cu2+, Mn2+, Zn2+, Na+, K+, Mg2+, Ba2+, and Ca2+ at 5 mM. These excellent characteristics made it a highly attractive candidate for industrial use.
Similar content being viewed by others
References
Yoshida, H. (1883) Chemistry of lacquer (urushi). J. Chem. Soc. 43: 472–486.
Alexandre, G. and I. B. Zhulin (2000) Laccases are widespread in bacteria. Trends Biotechnol. 18: 41–42.
Mayer, A. M. and R. C. Staples (2002) Laccase: New functions for an old enzyme. Phytochem. 60: 551–565.
Thurston, C. F. (1994) The structure and function of fungal laccases. Microbiol. 140: 19–26.
Hadzhiyska, H., M. Calafell, J. M. Gibert, J. M. Dagà, and T. Tzanov (2006) Laccase-assisted dyeing of cotton. Biotechnol. Lett. 28: 755–759.
Niladevi, K. N. and P. Prema (2008) Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour. Technol. 99: 4583–4589.
Zouari-Mechichi, H., T. Mechichi, A. Dhouib, S. Sayadi, A. T. Martínez, and M. J. Martínez (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: Decolorization of textile dyes by the purified enzyme. Enz. Microb. Technol. 39: 141–148.
Box, G. E. P. and D. W. Behnken (1960) Some new three level design for study of quantitative variables. Technometrics 2: 455–475.
Ryu, W. R., S. H. Shim, M. Y. Jang, Y. J. Jeon, K. K. Oh, and M. H. Cho (2000) Biodegradation of pentachlorophenol by white rot fungi underligninolytic and nonligninolytic Conditions. Biotechnol. Bioproc. Eng. 5: 211–214.
Zhang, H. B., Y. L. Zhang, F. Huang, P. J. Gao, and J. C. Chen (2009) Purification and characterization of a thermostable laccase with unique oxidative characteristics from Trametes hirsuta. Biotechnol. Lett. 31: 837–843.
Sharma, P., L. Singh, and N. Dilbaghi (2009) Optimization of process variables for decolorization of Disperse Yellow 211 by Bacillus subtilis using Box-behnken design. J. Hazard. Mater. 164: 1024–1029.
Palvannan, T. and P. Sathishkumar (2010) Production of laccase from Pleurotus florida NCIM 1243 using Plackett-Burman design and response surface methodology. J. Basic Microbiol. 50: 325–335.
Bourbonnais, R., M. G. Paice, I. D. Reid, P. Lanthier, and M. Yaguchi (1995) Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,20-azinobis (3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 61: 1876–1880.
Minussi, R. C., M. A. Miranda, J. A. Silva, C. V. Ferreira, H. Aoyama, S. Marangoni, D. Rotilio, G. M. Pastore, and N. Durán (2007) Purification, characterization and application of laccase from Trametes versicolor for colour and phenolic removal of olive mill wastewater in the presence of 1-hydroxybenzotriazole. Afr. J. Biotechnol. 6: 1248–1254.
Han, M. J., H. T. Choi, and H. G. Song (2005) Purification and characterization of laccase from the white rot fungus Trametes versicolor. J. Microbiol. 43: 555–560.
Forootanfar, H., M. A. Faramarzi, A. R. Shahverdi, and M. T. Yazdi (2011) Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresour. Technol. 102: 1808–1814.
Halaburgi, V. M., S. Sharma, M. Sinha, T. P. Singh, and T. B. Karegoudar (2011) Purification and characterization of a thermostable laccase from the ascomycetes Cladosporium cladosporioides and its applications. Proc. Biochem. 46: 1146–1152.
Coll, P. M., J. M. Fernandez-Abalos, J. R. Villanueva, R. Santamaria, and P. Perez (1993) Purification and characterization of a phenoloxidase (laccase) from the lignindegrading basidiomycete PM1(CECT 2971). Appl. Environ. Microbiol. 59: 2607–2613.
Jung, J., F. Xu, and K. Li (2002) Purification and characterization of laccase from wood-degrading fungus Trichophyton rubrum LKY-7. Enz. Microb. Technol. 30: 161–168.
Nakade, K., Y. Nakagawa, A. Yano, T. Sato, and Y. Aakamoto (2010) Characterization of an extracellular laccase, PbLac1, purified from Polyporus brumalis. Fungal Biol. 114: 609–618.
Liu, L. H., Z. W. Lin, T. Zheng, L. Lin, C. Q. Zheng, Z. X. Lin, S. H. Wang, and Z. H. Wang (2009) Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enz. Microb. Technol. 44: 426–433.
Murugesan, K., M. Arulmani, I. H. Nam, Y. M. Kim, Y. S. Chang, and P. T. Kalaichelvan (2006) Purification and characterization of laccase produced by a white rot fungus Pleurotus sajorcaju under submerged culture condition and its potential in decolorization of azo dyes. Appl. Microbiol. Biotechnol. 72: 939–946.
Levin, L., A. Viale, and A. Forchiassin (2003) Degradation of organic pollutants by the white rot basidiomycete Trametes trogii. Int. Biodet. Biodeg. 52: 1–5.
Baldrian, P. (2006) Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 30: 215–242.
Schückel, J., A. Matura, and K. H. V. Pée (2011) One-copper laccase-related enzyme from Marasmius sp.: Purification, characterization and bleaching of textile dyes. Enz. Microb. Technol. 48: 278–284.
Wang, Z. X., Y. J. Cai, X. R. Liao, G. J. Tao, Y. Y. Li, F. Zhang, and D. B. Zhang (2010) Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Proc. Biochem. 45: 1720–1729.
Xu, F. (1996) Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochem. 35: 7608–7614.
Liers, C., R. Ullrich, M. Pecyna, D. Schlosser, and M. Hofrichter (2007) Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enz. Microb. Technol. 41: 785–793.
Reiss, R., J. Ihssen, and L. Thöny-Meyer (2011) Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 11: 9.
D’souza-Ticlo, D., D. Sharma, and C. Raghukumar (2009) A thermostable Metal-tolerant laccase with bioremediation potential from a Marine-derived fungus. Mar. Biotechnol. 11: 725–737.
Niku-Paavola, M. L., R. Fagerström, K. Kruus, and L. Viikari (2004) Thermostable laccases produced by a white-rot fungus from Peniophora species. Enz. Microb. Technol. 35: 100–102.
Palmieri, G., P. Giardina, C. Bianco, A. Scaloni, A. Capasso, and G. Sannia (1997) A novel white laccase from Pleurotus ostreatus. J. Biol. Chem. 272: 31301–31307.
Asgher, M., H. N. Bhatti, M. Ashraf, and R. L. Legge (2008) Recent developments in biodegradation of industrial pollutants by white-rot fungi and their enzyme system. Biodegradation 19: 771–783.
Wong, K. K. Y., J. D. Richardson, and S. D. Mansfield (2000) Enzymatic treatment of mechanical pulp fibres for improving papermaking properties. Biotechnol. Prog. 16: 1025–1029.
Ko, E. M., Y. E. Leem, and H. T. Choi (2001) Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl. Microbiol. Biotechnol. 57: 98–102.
Telke, A. A., A. A. Kadam, S. S. Jagtap, J. P. Jadhav, and S. P. Govindwar (2010) Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol. Bioproc. Eng. 15: 696–703.
Farnet, A. M., G. Gil, and E. Ferre (2008) Effects of pollutants on laccase activities of Marasmius quercophilus, a white-rot fungus isolated from a Mediterranean schlerophyllous litter. Chemosphere 70: 895–900.
Guo, L. Q., S. X. Lin, X. B. Zheng, Z. R. Huang, and J. F. Lin (2011) Production, purification and characterization of a thermostable laccase from a tropical white-rot fungus. World J. Microbiol. Biotechnol. 27: 731–735.
Casas, N., T. Parella, T. Vicent, G. Caminal, and M. Sarrà (2009) Metabolites from the biodegradation of triphenylmethane dyes by Trametes versicolor or laccase. Chemosphere 75: 1344–1349.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhang, H., Cao, M. et al. Production of a thermostable metal-tolerant laccase from Trametes versicolor and its application in dye decolorization. Biotechnol Bioproc E 16, 1027–1035 (2011). https://doi.org/10.1007/s12257-011-0129-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-011-0129-0