Summary
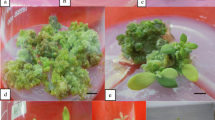
An in vitro method was developed for high-frequency shool regeneration and continuous production of rapid-cycling B. oleracea in large numbers. The high regenerative capacity was tissue-dependent. Developmental polarity (apical and basal ends) of the explants appeared to play a regulatory role in shoot morphogenesis in this system. High-frequency shoot regeneration was obtained with N6-benzyladenine or thidiazuron-supplemented media. Delayed and reduced regenerative ability of cultures in air-tight vessels and the dramatic suppression of shoot regeneration in internodal explants by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid implicate a possible involvement of ethylene in shoot morphogenesis in this species. Rotting of regenerated shoots of B. oleracea occurred readily on α-naphthaleneacetic acid-supplemented media. Rooted plantlets were successfully established in soil and developed normal fertile flowers and viable seeds.
Similar content being viewed by others
References
Arumuganathan, K.; Earle, E. D. Nuclear DNA content of some important plant species. Plant Mol. Biol. Rep. 9:208–218; 1991.
Aslam, F. N.; MacDonald, M. V.; Ingram, D. S. Rapid-cycling Brassica species: anther culture potential of B. campestris L. and B. napus L. New Phytol. 115:1–9; 1990.
Bajaj, Y. P. S.; Nietsch, P. In vitro propagation of red cabbage (Brassica oleracea L. var capitata). J. Exp. Bot. 26:883–890; 1975.
Berthomieu, P.; Jouanin, L. Transformation of rapid cycling cabbage (Brassica oleracea var capitata) with Agrobacterium rhizogenes. Plant Cell Rep. 11:334–338; 1992.
Chi, G. L.; Pua, E. C.; Goh, C. J. Role of ethylene on de novo shoot regeneration from cotyledonary explants of Brassica campestris spp. pekinensis (Lour) Olsson in vitro. Plant Physiol. 96:178–183; 1991.
Fosket, D. E. Plant growth and development. California: Academic Press; 1994:363–369.
Hansen, L. N.; Earle, E. D. Regeneration of plants from protoplasts of rapid cycling Brassica oleracea L. Plant Cell Rep. 13:335–339; 1994.
Kik, C.; Zaal, M. A. C. M. Protoplast regeneration from Brassica oleracea “rapid cycling’ and other varieties. Plant Cell Tiss. Organ Cult. 35:107–114; 1993.
Kumar, P. P.; Lakshmanan, P.; Thorpe, T. A. Regulation of morphogenesis in plant tissue culture by ethylene. In Vitro Cell Dev. Biol. Plant 34:94–103; 1998.
Lakshmanan, P.; Ng, S. K.; Loh, C. S.; Goh, C. J. Auxin, cytokinin and ethylene differentially regulate specific developmental states associated with shoot bud morphogenesis in leaf tissues of mangosteen (Garcinia mangostana L.) cultured in vitro. Plant Cell Physiol. 38:59–64; 1997.
Larkin, P. J.; Scowcroft, W. R. Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60:197–214; 1981.
Leung, H.; Williams, P. Cytoplasmic male sterile Brassica campestris breeding lines with resistance to clubroot, turnip mosaic, and downy mildew. HortScience 18:774–775; 1983.
Loudon, P. T.; Nelson, R. S.; Ingram, D. Studies of protoplast culture and plant regeneration from commercial and rapid-cycling Brassica species. Plant Cell Tiss. Organ Cult. 19:213–224; 1989.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15:473–497; 1962.
Narasimhulu, S. B.; Kirti, P. B.; Mohapatra, T.; Prakash, S.; Chopra, V. L. Shoot regeneration in stem explants and its amenability to Agrobacterium tumefaciens mediated gene transfer in Brassica carinata. Plant Cell Rep. 11:359–362; 1992.
Paterson, K. E. Polarity of regeneration in excised leaves of Crassula argentea. 1. A role of auxin. Can. J. Bot. 61:1058–1063; 1983.
Pua, E. C. Cellular and molecular aspects of ethylene on plant morphogenesis of recalcitrant Brassica species in vitro. Bot. Bull. Acad. Sinica 34:191–209; 1993.
Bood, S.; Hedden, P. Convergent pathways of gibberellin A1 biosynthesis in Brassica. Plant Growth Reg. 15:241–246; 1994.
Stringam, G. R. Regeneration in stem explants of haploid rapessed (Brassica napus L.) Plant Sci. Lett. 9:115–119; 1977.
Teo, W.; Lakshmanan, P.; Kumar, P.; Goh, C. J.; Swarup, S. Direct shoot formation and plant regeneration from cotyledon explants of rapid-cycling Brassica rapa. In Vitro Cell. Dev. Biol. Plant 33:288–292; 1997.
Williams, P. H.; Hill, C. B. Rapid-cycling populations of Brassica. Science 232:1385–1390; 1986.
Wong, R. S. C.; Zee, S. Y.; Swanson, E. B. Isolated microspore culture of Chinese flowering cabbage (Brassica campestris spp. parachinensis) Plant Cell Rep. 15:396–400; 1996.
Yang, M. Z.; Jia, S. R.; Pua E. C. High frequency of plant regeneration from hypocotyl explants of Brassica carinata. Plant Cell Tiss. Organ Cult. 24:79–82; 1991.
Zar, J. H. Biostatistical analysis 2nd edn. Englewood Cliffs, NJ: Prentice-Hall; 1984:718.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, PK., Lakshmanan, P. & Swarup, S. High-frequency direct shoot regeneration and continuous production of rapid-cycling Brassica oleracea in vitro . In Vitro Cell.Dev.Biol.-Plant 37, 592–598 (2001). https://doi.org/10.1007/s11627-001-0104-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11627-001-0104-0