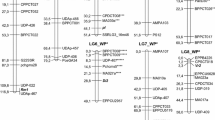
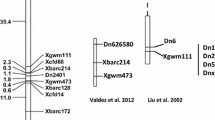
Abstract
The green peach aphid (GPA), Myzus persicae (Sulzer), is a widespread pest insect that significantly reduces yield in peach orchards [Prunus persica (L.) Batsch]. Chemical control of the GPA population in the orchards showed little efficiency because of the development of resistance to most classes of insecticides. Biological control partially gave convincing results. Breeding for resistant peach cultivars is therefore a serious option to take into account for the development of sustainable pest management. Among the few available resistance cultivars, the rootstock peach “Rubira®” shows a strong induced antixenosis-type GPA resistance. This was demonstrated segregating as a single dominant gene. In order to investigate the genetic basis of resistance and develop molecular tools useful in breeding programs, a F2 population derived from “Rubira®” also segregating for leaf color was grown and scored for GPA resistance under contrasted environmental conditions. An SSR-based genetic linkage map composed of 120 SSR loci spanned over a distance of 497.8 cM was then established. The GPA resistance mapped to a single locus at the bottom end of linkage group 1. We propose to name Rm2 the dominant allele of the underlying gene. Additionally, a reciprocal translocation was identified near the Gr gene controlling leaf color. The red-leaf parent “Rubira®” was demonstrated responsible for the translocation. This study provides the basis for future molecular analysis for the use of Rm2 in peach breeding programs against GPA in peach orchards.



Similar content being viewed by others
References
Aranzana MJ, Garcia-Mas J, Carbó J, Arús P (2002) Development and variability analysis of microsatellite markers in peach. Plant Breed 121:87–92
Aranzana MJ, Pineda A, Cosson P, Dirlewanger E, Ascasibar J, Cipriani G, Ryder CD, Testolin R, Abbott A, King GJ, Iezzoni AF, Arús P (2003) A set of simple-sequence repeat (SSR) markers covering the Prunus genome. Theor Appl Genet 106:819–825
Arús P, Yamamoto T, Dirlewanger E, Abbott AG (2005) Synteny in the Rosaceae. In: Janick J (ed) Plant breeding reviews. Wiley, New York, pp 175–211
Barker E, Holaschke M, Fulton A, Evans K, Powell G (2007) Effects of kaolin particle film on Myzus persicae (Hemiptera: Aphididae) behaviour and performance. Bull Entomol Res 97:455–460
Bernatzky R, Tanksley SD (1986) Genetics of actin-related sequences in tomato. Theor Appl Genet 72:314–321
Blake MA (1937) Progress in peach breeding. Proc Am Soc Hortic Sci 35:49–53
Blackman RL, Eastop VF (2000) Aphids on the world's crops. An identification and information guide, 2nd edn. Wiley, Chichester
Blenda A, Verde I, Georgi L, Reighard G, Forrest S, Muñoz-Torres M, Baird W, Abbott A (2007) Construction of a genetic linkage map and identification of molecular markers in peach rootstocks for response to peach tree short life syndrome. Tree Genet Genomes 3:341–350
Bus VGM, Chagné D, Basset HCM, Bowatte D, Calenge F, Celton JM, Durel CE, Malone MT, Patocchi A, Ratanunga AC, Rikkerink AHA, Tustin DS, Zhou J, Gardiner SE (2008) Genome mapping of three major resistance genes to wooly apple aphid (Eriosoma lanigerum Hausm.). Tree Genet Genomes 4:223–236
Bus VGM, Bassett HCM, Bowatte D, Chagné D, Ranatunga CA, Ulluwishewa D, Wiedow C, Gardiner SE (2010) Genome mapping of an apple scab, a powdery mildew and a woolly apple aphid resistance gene from open-pollinated Mildew Immune Selection. Tree Genet Genomes 6:477–487
Cantini C, Iezzoni AF, Lamboy WF, Boritzki M, Struss D (2001) DNA fingerprinting of tetraploid cherry germplasm using SSRs. J Am Soc Hort Sci 126:205–209
Cevik V, King GJ (2002) High resolution genetic analysis of the Sd-1 aphid resistance locus in Malus spp. Theor Appl Genet 105:346–354
Clarke JB, Tobutt KR (2003) Development and characterization of polymorphic microsatellites from Prunus avium “Napoleon”. Mol Ecol Notes 3:578–580
Cipriani G, Lot G, Huang WG, Marrazzo MT, Peterlunger E, Testolin R (1999) AC/GT and AG/CT microsatellite repeats in peach [Prunus persica (L) Batsch]: isolation, characterisation and cross-species amplification in Prunus. Theor Appl Genet 99:65–72
Cravedi P, Cervato P (1997) Resistance to insecticides of the green peach aphid and Integrated Fruit Production guidelines. IOBC Bull 20(6):75–77
Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S (2004) Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic Biochem Physiol 78:83–92
Decroocq V, Favé MG, Hagen L, Bordenave L, Decroocq S (2003) Development and transferability of apricot and grape EST microsatellite markers across taxa. Theor Appl Genet 106:912–922
Decroocq V, Foulongne M, Lambert P, Le Gall P, Mantin C, Pascal T, Schurdi-Levraud V, Kervella J (2005) Analogues of virus resistance genes map to QTLs for resistance to sharka disease in Prunus davidiana. Mol Genet Genomics 272:680–689
Devonshire AL, Field LM, Foster SP, Moores GD, Williamson MS, Blackman LR (1998) The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae. Philos Trans R Soc Lond B Biol Sci 353:1677–1684
Dirlewanger E, Cosson P, Tavaud M, Aranzana MJ, Poizat C, Zanetto A, Arús P, Laigret F (2002) Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor Appl Genet 105:127–138
Dirlewanger E, Cosson P, Howad W, Capdeville G, Bosselut N, Claverie M, Voisin R, Poizat C, Lafargue B, Baron O (2004) Microsatellite genetic linkage maps of myrobalan plum and an almond-peach hybrid—location of root-knot nematode resistance genes. Theor Appl Genet 109:827–838
Dirlewanger E, Cosson P, Boudehri K, Renaud C, Capdeville G, Tauzin Y, Laigret F, Moing A (2006) Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet Genomes 3:1–13
Dogimont CA, Bendahmane A, Pitrat M et al. (2004) Gène de résistance à Aphys gossypii. World patent WO2004072109
Dondini L, Lain O, Geuna F, Banfi R, Gaiotti F, Tartarini S, Bassi D, Testolin R (2007) Development of a new SSR-based linkage map in apricot and analysis of synteny with existing Prunus maps. Tree Genet Genomes 3:239–249
Downey LD, Iezzoni AF (2000) Polymorphic DNA markers in black cherry are identified using sequences from sweet cherry, peach, and sour cherry. J Am Soc Hort Sci 125:76–80
Evans K, Govan CL, Fernández-Fernández F (2008) A new gene for resistance to Dysaphis pyri in pear and identification of flanking microsatellite markers. Genome 51:1026–1031
Fos A, Massonié G (1993) Transmission expérimentale du virus de la sharka par Brachycaudus persicae Passerini. Agronomie 13(6):515–518
Foster SP, Devine G, Devonshire AL (2007) Insecticide resistance. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CABI, Oxfordshire, pp 261–285
Foulongne M, Pascal T, Arús P, Kervella J (2003a) The potential of Prunus davidiana for introgression into peach [Prunus persica (L.) Batsch] assessed by comparative mapping. Theor Appl Genet 107:227–238
Foulongne M, Pascal T, Pfeiffer F, Kervella J (2003b) QTLs for powdery mildew resistance in peach × Prunus davidiana crosses: consistency across generations and environment. Mol Breed 12:33–50
Gao L, Klingler JP, Anderson JP, Edwards OR, Singh KB (2008) Characterization of pea aphid resistance in Medicago truncatula. Plant Physiol 146:996–1009
Gentz MC, Murdoch G, King GF (2010) Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest management. Biol Control 52:208–215
Goggin FL, Shah G, Williamson VM, Ullman DE (2004) Developmental regulation of Mi-mediated aphid resistance is independent of Mi-1.2 transcript levels. Mol Plant Microbe Interact 17:532–536
Hagen LS, Chaib J, Fady B, Decroocq V, Bouchet JP, Lambert P, Audergon JM (2004) Genomic and cDNA microsatellites from apricot (Prunus armeniaca L.). Mol Ecol Notes 4:742–745
Hesse CO (1975) Peaches. In: Jannick J, Moore JN (eds) Advances in fruit breeding. Purdue University Press, West Lafayette, pp 285–335
Hill CB, Li Y, Hartman GL (2006) A single dominant gene for resistance to the soybean aphid in the soybean cultivar Dowling. Crop Sci 46:1601–1605
Howad W, Yamamoto T, Dirlewanger E, Testolin R, Cosson P, Cipriani G, Monforte AJ, Georgi L, Abbott AG, Arus P (2005) Mapping with a few plants: using selective mapping for microsatellite saturation of the Prunus reference map. Genetics 171:1305–1309
Jáuregui B, de Vicente MC, Messeguer R, Felipe A, Bonnet A, Salesses G, Arús P (2001) A reciprocal translocation between “Garfi” almond and “Nemared” peach. Theor Appl Genet 102:1169–1176
Joobeur T, Periam N, de Vicente MC, King GJ, Arús P (2000) Development of a second generation linkage map for almond using RAPD and SSR markers. Genome 43:649–655
Kervella J, Pascal T, Pfeiffer F, Dirlewanger E (1998) Breeding for multiresistance in peach tree. Acta Hort 465:177–184
Kfoury L, Massonié G (1995) Caractéristiques de la résistance du cultivar de pêcher Rubira® à Myzus persicae Sulzer. Agronomie 15(5):277–284
Klingler J, Creasy R, Gao L, Nair RM, Calix AS, Spattford Jacob H, Edwards OR, Singh KB (2005) Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol 137:1445–1455
Klingler J, Edwards OR, Singh KB (2007) Independent action and contrasting phenotypes of resistance genes against spotted alfalfa aphid and bluegreen aphid in Medicago truncatula. New Phytol 173:630–640
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Kruglyak L, Lander ES (1995) A non-parametric approach for mapping quantitative trait loci. Genetics 139:1421–1428
Lalli DA, Decroocq V, Blenda AV, Shurdi-Levraud V, Garay L, Le Gall O, Damsteegt V, Reighard GL, Abbott AG (2005) Identification and mapping of resistance gene analogs (RGAs) in Prunus: a resistance map for Prunus. Theor Appl Genet 111:1504–1513
Lambert P, Hagen LS, Arus P, Audergon JM (2004) Genetic linkage maps of two apricot cultivars (Prunus armeniaca L.) compared with the almond ‘Texas’ x peach ‘Earlygold’ reference map for Prunus. Theor Appl Genet 108:1120–1130
Lambert P, Dicenta F, Rubio M, Audergon JM (2007) QTL analysis of resistance to sharka disease in the apricot (Prunus armeniaca L.) ‘Polonais’ × ‘Stark Early Orange’ F1 progeny. Tree Genet Genomes 3:299–309
Lambert P, Sauge MH, Poëssel JL, Pascal T (2008) Pyramiding mono and polygenic resistances is one strategy to provide lasting control of the resistance to the green peach aphid and powdery mildew in peach. Fourth Rosaceae Conference, Pucon, 16–19 March 2008
Li Y, Hill C, Carlson S, Diers B, Hartman G (2007) Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group M. Mol Breed 19:25–34
Lincoln SE, Daly MJ, Lander ES (1992) Constructing genetic maps with Mapmaker/exp 3.0, 3rd edn. Whitehead Institute Technical Report, Cambridge
Liu XM, Smith CM, Friebe BR, Gill BS (2005) Molecular mapping and allelic relationships of Russian wheat aphid-resistance genes. Crop Sci 45(6):2273–2280
Lopes MS, Sefc KM, Laimer M, Da Câmara MA (2002) Identification of microsatellite loci in apricot. Mol Ecol Notes 2:24–26
Marandel G, Pascal T, Candresse T, Decroocq V (2009) Quantitative resistance to Plum pox virus in Prunus davidiana P1908 linked to components of the eukaryotic translation initiation complex. Plant Pathol 58:425–435
Massonié G, Maison P, Monet R, Grasselly C (1982) Résistance au puceron vert du pêcher, Myzus persicae Sulzer (Homoptera: Aphididae) chez Prunus persica (L) Batsch et d'autres espèces de Prunus. Agronomie 2:63–70
Mazzoni E, Cravedi P (2002) Analysis of insecticide-resistant Myzus persicae (Sulzer) populations collected in Italian peach orchards. Pest Manag Sci 58:975–980
Messina R, Lain O, Marrazzo MT, Cipriani G, Testolin R (2004) New set of microsatellite loci isolated in apricot. Mol Ecol Notes 4:432–434
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root-knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10:1307–1319
Mnejja M, Garcia-Mas J, Howad W, Badenes ML, Arús P (2004) Simple-sequence repeat (SSR) markers of Japanese plum (Prunus salicina Lindl.) are highly polymorphic and transferable to peach and almond. Mol Ecol Notes 4:163–166
Mnejja M, Garcia-Mas J, Howad W, Arús P (2005) Development and transportability across Prunus species of 42 polymorphic almond microsatellites. Mol Ecol Notes 5:531–535
Monet R, Massonié G (1994) Déterminisme génétique de la résistance au puceron vert (Myzus persicae) chez le pêcher. Résultats complémentaires. Agronomie 2:177–182
Ogundiwin EA, Peace CP, Gradziel TM, Parfitt DE, Bliss FA, Crisosto CH (2009) A fruit quality gene map of Prunus. BMC Genomics 10:587
Pascal T, Pfeiffer F, Kervella J, Lacroze JP, Sauge MH (2002) Inheritance of green peach aphid resistance in the peach cultivar “Rubira®”. Plant Breed 121:459–461
Peck DC (2009) Long-term effects of imidacloprid on the abundance of surface and soil-active nontarget fauna in turf. Agr Forest Entomol 11:405–419
Pitrat M, Lecoq H (1980) Inheritance of resistance to cucumber mosaic virus transmission by Aphis gossypii in Cucumis melo. Phytopathology 70:958–961
Poëssel JL, Corre M, Kervella J et al. (2002) Increase in phenolic content in the resistant peach cultivar “Rubira®” infested by the green peach aphid, Myzus persicae. In: El Hadrami, I (ed) XXI Int Conf on Polyphenols—Groupe Polyphenols Publisher, Montpellier (FRA) vol 1:131–132
Rajapakse S, Belthoff LE, He G, Estager AE, Scorza R, Verde I, Ballard RE, Baird WV, Callahan A, Monet R, Abbott AG (1995) Genetic linkage mapping in peach using morphological, RFLP and RAPD markers. Theor Appl Genet 90:503–510
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VW (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci U S A 95:9750–9754
Rubio M, Pascal T, Bachellez A, Lambert P (2010) Quantitative trait loci analysis of Plum pox virus resistance in Prunus davidiana P1908: new insights on the organization of genomic resistance regions. Tree Genet Genomes 6:291–304
Sauge MH, Kervella J, Rahbé Y (1998a) Probing behaviour of the green peach aphid Myzus persicae on resistant Prunus genotypes. Entomol Exp Appl 89:223–232
Sauge MH, Kervella J, Pascal T (1998b) Settling behaviour and reproductive potential of the green peach aphid Myzus persicae on peach varieties and a related wild Prunus. Entomol Exp Appl 89:233–242
Sauge MH, Lacroze JP, Poëssel JL, Pascal T, Kervella J (2002) Induced resistance by Myzus persicae in the peach cultivar “Rubira®”. Entomol Exp Appl 102:29–37
Sauge MH, Pascal T, Lacroze JP, Pfeiffer F, Kervella J et al (2004) Mapping of a genetic factor of partial resistance to Myzus persicae in the wild peach Prunus davidiana, that impedes phloem sap ingestion by the aphid. In: Simon JC, Dedryver CA, Rispe C (eds) Aphids in a new millennium. INRA Editions, Versailles, pp 499–505
Sauge MH, Mus F, Lacroze JP, Pascal T, Kervella J, Poëssel JL (2006) Genotypic variation in induced resistance and induced susceptibility in the peach-Myzus persicae aphid system. Oikos 113:305–313
Sicard O, Marandel G, Soriano JM, Lalli DA, Lambert P, Salava J, Badenes M, Abbott A, Decroocq V (2008) Flanking the major Plum pox virus resistance locus in apricot with co-dominant markers (SSRs) derived from candidate resistance genes. Tree Genet Genomes 4:359–365
Sosinski B, Gannavarapu M, Hager LD, Beck LE, King GJ, Ryder CD, Rajapakse S, Baird WV, Ballard RE, Abbott AG (2000) Characterization of microsatellite markers in peach (Prunus persica L. Batsch). Theor Appl Genet 101:421–42
Tagu D, Klingler JP, Moya A, Simon JC (2008) Early progress in aphid genomics and consequences for plant–aphid interactions studies. MPI 21(6):701–708
Testolin R, Marrazzo T, Cipriani G, Quarta R, Verde I, Dettori M, Pancaldi M, Sansavini S (2000) Microsatellite DNA in peach (Prunus persica L. Batsch) and its use in fingerprinting and testing the genetic origin of cultivars. Genome 43:512–520
Testolin R, Messina R, Lain O, Marrazzo MT, Huang WG, Cipriani G (2004) Microsatellites isolated in almond from an AC-repeat enriched library. Mol Ecol Notes 4:459–461
Van Ooijen JW (2004) MapQTL® 5, Software for the mapping of quantitative trait loci in experimental populations. Kyazma BV, Wageningen
Verde I, Lauria M, Dettori MT, Vendramin E, Balconi C, Micali S, Wang Y, Marrazzo MT, Cipriani G, Hartings H, Testolin R, Abbott AG, Motto M, Quarta R (2005) Microsatellite and AFLP markers in the Prunus persica [L. (Batsch)] × P. ferganensis BC1 linkage map: saturation and coverage improvement. Theor Appl Genet 111:1013–1021
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78
Wroblewski T, Piskurewicz U, Tomczak A, Ochoa O, Michelmore RW (2007) Silencing of the major family of NBS-LRR-encoding genes in lettuce results in the loss of multiple resistance specificities. Plant J 51:803–818
Yamamoto T, Mochida K, Imai T, Shi YZ, Ogiwara I, Hayashi T (2002) Microsatellite markers in peach [Prunus persica (L.) Batsch] derived from an enriched genomic and cDNA libraries. Mol Ecol Notes 2:298–301
Yamamoto T, Hayashi T (2002) New root-knot nematode resistance genes and their STS markers in peach. Sci Hortic 96:81–90
Yamamoto T, Yamaguchi M, Hayashi T (2005) An integrated genetic linkage map of peach by SSR, STS, AFLP and RAPD. J Jpn Soc Hort Sci 74:204–213
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Acknowledgments
The authors acknowledge A. Bachellez, E. Lecerf, and X. Titeca for the technical assistance and the International Peach Initiative Consortium (IPGI) for the early release of the peach genome sequence v1.0. All experiments described in this paper comply with the current laws in the European Union.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Abbott
Rights and permissions
About this article
Cite this article
Lambert, P., Pascal, T. Mapping Rm2 gene conferring resistance to the green peach aphid (Myzus persicae Sulzer) in the peach cultivar “Rubira®”. Tree Genetics & Genomes 7, 1057–1068 (2011). https://doi.org/10.1007/s11295-011-0394-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-011-0394-2