Abstract
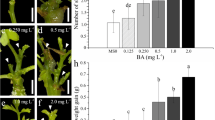
Kohlrabi (Brassica oleracea var. gongylodes) cultivars Vienna Purple (VP) and Vienna White (VW) were tested for their ability of de novo organogenesis in vitro. Root, cotyledon, hypocotyl explants and intact seedlings were cultivated on Murashige and Skoog (MS) media supplemented with different cytokinins: benzyladenine (BA), thidiazuron (TDZ), trans- or cis-zeatin. All tested cytokinins, including cis-zeatin, induced shoot regeneration from hypocotyl explants and intact seedlings, with seedlings being most successful for regeneration efficiency and viability of regenerated shoots in both cultivars. The highest frequency of shoot regeneration was achieved on MS with BA (60 %) or TDZ (50 %) for VP; and with BA (50 %), TDZ (47.5 %) or transZ (37.5 %) for VW. Measurements of the endogenous cytokinin and indole-3-acetic acid (IAA) contents in both hypocotyl explants and seedlings with regenerated shoots (HRSs and SRSs) suggested that the observed differences in organogenic response between these two types of explants were related to their cytokinin and IAA contents. HRSs generally exhibited elevated amounts of total cytokinins, while SRSs displayed a higher IAA/bioactive cytokinins ratio. Shoots regenerated from seedlings were further successfully multiplicated on a medium supplemented with BA (0.5 mg L−1). The rooting potential of multiplicated shoots was tested on media supplemented with 2 or 4 mg L−1 indole-3-butyric acid (IBA), with the higher concentration of IBA leading to more efficient rooting. Rooted plantlets were successfully planted into soil and flow cytometric analysis did not reveal ploidy variations, indicating that the described protocol is fast and efficient for kohlrabi regeneration.







Similar content being viewed by others
Abbreviations
- BA:
-
N 6-benzyladenine
- cisZ:
-
cis-Zeatin
- cisZR:
-
cis-Zeatin-9-riboside
- cisZ7G:
-
cis-Zeatin-7-glucoside
- cisZ9G:
-
cis-Zeatin-9-glucoside
- cisZOG:
-
cis-Zeatin-O-glucoside
- cisZROG:
-
cis-Zeatin-9-riboside-O-glucoside
- cisZRMP:
-
cis-Zeatin-9-riboside-5′-monophosphate
- CK:
-
Cytokinin
- DNSO:
-
De novo shoot organogenesis
- DHZ:
-
Dihydrozeatin
- DHZR:
-
Dihydrozeatin-9-riboside
- DHZ7G:
-
Dihydrozeatin-7-glucoside
- DHZ9G:
-
Dihydrozeatin-9-glucoside
- DHZOG:
-
Dihydrozeatin-O-glucoside
- DHZROG:
-
Dihydrozeatin-9-riboside-O-glucoside
- DHZRMP:
-
Dihydrozeatin-9-riboside-5′-monophosphate
- HRS:
-
Hypocotyl with regenerated shoots
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- iP:
-
N 6-(∆2-isopentenyl)adenine
- iPR:
-
N 6-(∆2-isopentenyl)adenosine
- iP7G:
-
N 6-(∆2-isopentenyl)adenine-7-glucoside
- iP9G:
-
N 6-(∆2-isopentenyl)adenine-9-glucoside
- iPRMP:
-
N 6-(∆2-isopentenyl)adenosine -5′-monophosphate
- PGR:
-
Plant growth regulator
- SRS:
-
Seedling with regenerated shoots
- TDZ:
-
Thidiazuron
- transZ:
-
trans-Zeatin
- transZR:
-
trans-Zeatin-9-riboside
- transZ7G:
-
trans-Zeatin-7-glucoside
- transZ9G:
-
trans-Zeatin-9-glucoside
- transZOG:
-
trans-Zeatin-O-glucoside
- transZROG:
-
trans-Zeatin-9-riboside-O-glucoside
- transZRMP:
-
trans-Zeatin-9-riboside-5′-monophosphate
- VP:
-
Vienna Purple cultivar
- VW:
-
Vienna White cultivar
References
Abbasi BH, Khan M, Guo B, Bokhari SA, Khan MA (2011) Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tissue Organ Cult 105:337–344
Ahmadabadi M, Bock R (2010) Development of a highly responsive leaf-based regeneration system for Peperomia species. Turk J Bot 34:329–334
Akasaka-Kennedy Y, Yoshida H, Takahata Y (2005) Efficient plant regeneration from leaves of rape seed (Brassica napus L.): influence of AgNO3 and genotype. Plant Cell Rep 24:649–654
Aremu AO, Plačková L, Bairu MW, Novák O, Plíhalová L, Doležal K, Finnie JF, Van Staden J (2014a) How does exogenously applied cytokinin type affect growth and endogenous cytokinins in micropropagated Merwilla plumbea? Plant Cell Tissue Organ Cult 118:245–256
Aremu AO, Plačková L, Bairu MW, Novák O, Szüčová L, Doležal K, Finnie JF, Van Staden J (2014b) Endogenous cytokinin profiles of tissue-cultured and acclimatized ‘Williams’ bananas subjected to different aromatic cytokinin treatments. Plant Sci 214:88–98
Auer CA, Cohen JD, Laloue M, Cooke TJ (1992) Comparison of benzyl adenine metabolism in two Petunia hybrida lines differing in shoot organogenesis. Plant Physiol 98:1035–1041
Bajguz A, Piotrowska A (2009) Conjugates of auxin and cytokinin. Phytochemistry 70:957–969
Baroja-Fernández E, Aguirreolea J, Martínková H, Hanuš J, Strnad M (2002) Aromatic cytokinins in micropropagated potato plants. Plant Physiol Biochem 40:217–224
Bassil NV, Mok DWS, Mok MC (1993) Partial purification of a cis-trans-isomerase of zeatin from immature seed of Phaseolus vulgaris L. Plant Physiol 102:867–872
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Bhalla PL, de Weerd N (1999) In vitro propagation of cauliflower, Brassica oleracea var. botrytis for hybrid seed production. Plant Cell Tissue Organ Cult 56:89–95
Bouza L, Jacques M, Sotta B, Miginiac E (1993) The differential effect of N6-benzyl-adenine and N6-(Δ2-isopentenyl)-adenine on in vitro propagation of Paeonia suffruticosa Andr. is correlated with different hormone contents. Plant Cell Rep 12:593–596
Bretagne B, Chupeau MC, Chupeau Y, Fouilloux G (1994) Improved flax regeneration from hypocotyls using thidiazuron as a cytokinin source. Plant Cell Rep 14:120–124
Brugière N, Jiao S, Hantke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE (2003) Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiol 132:1228–1240
Cardoza V, Stewart CN (2004) Brassica biotechnology: progress in cellular and molecular biology. In Vitro Cell Dev Biol Plant 40:542–551
Centeno ML, Rodríguez A, Feito I, Fernández B (1996) Relationship between endogenous auxin and cytokinin levels and morphogenic responses in Actinia deliciosa tissue cultures. Plant Cell Rep 16:58–62
Che P, Gingerich DJ, Lall S, Howell SH (2002) Global and hormone-induced gene expression changes during shoot development in Arabidopsis. Plant Cell 14:2771–2785
Che P, Lall S, Nettleton D, Howell SH (2006) Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol 141:620–637
Cheng ZJ, Wang L, Sun W, Zhang Y, Zhou C, Su YH, Li W, Sun TT, Zhao XY, Li XG, Cheng Y, Zhao Y, Xie Q, Zhang XS (2013) Pattern of auxin and cytokinin responses for shoot meristem induction results from the regulation of cytokinin biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol 161:240–251
Chikkala VRN, Nugent G, Dix P, Stevenson T (2009) Regeneration from leaf explants and protoplasts of Brassica oleracea var. botrytis (cauliflower). Sci Hortic 119:330–334
Cogbill S, Faulcon T, Jones G, McDaniel M, Harmon G, Blackmon R, Young M (2010) Adventitious shoot regeneration from cotyledonary explants of rapid-cycling fast plants of Brassica rapa L. Plant Cell Tissue Organ Cult 101:127–133
Coleman GD, Ernst SG (1989) In vitro shoot regeneration of Populus deltoids: effect of cytokikin and genotype. Plant Cell Rep 8:459–462
Ćosić T, Vinterhalter B, Vinterhalter D, Mitić N, Cingel A, Savić J, Bohanec B, Ninković S (2013) In vitro plant regeneration from immature zygotic embryos and repetitive somatic embryogenesis in kohlrabi (Brassica oleracea var. gongylodes). In Vitro Cell Dev Biol Plant 49:294–303
Cuesta C, Rodriguez A, Centeno ML, Ordas RJ, Fernandez B (2009) Caulogenic induction in cotyledons of tone pine (Pinus pinea): relationship between organogenic response and benzyladenine trends in elected families. J Plant Physiol 166:1162–1171
Cuesta C, Novák O, Ordás RJ, Fernández B, Strnad M, Doležal K, Rodríguez A (2012) Endogenous cytokinin profiles and their relationships to between-family differences during adventitious caulogenesis in Pinus pinea cotyledons. J Plant Physiol 169:1830–1837
Dai XG, Shi XP, Ye YM, Fu Q, Bao MZ (2009) High frequency plant regeneration from cotyledon and hypocotyl explants of ornamental kale. Biol Plant 53:769–773
Dobrev P, Kamínek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A 950:21–29
Dobrev P, Havlíček L, Vágner M, Malbeck J, Kamínek M (2005) Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatograophy. J Chromatogr A 1075:159–166
Duclercq J, Sangwan-Norreel B, Catterou M, Sangwan RS (2011) De novo shoot organogenesis: from art to science. Trends Plant Sci 16:597–606
Dwivedi S, Vaňková R, Motyka V, Herrera C, Žižková E, Auer C (2010) Characterization of Arabidopsis thaliana mutant ror-1 (roscovitine-resistant) and its utilization in understanding of the role of cytokinin N-glucosylation pathway in plants. Plant Growth Regul 61:231–242
Eklöf S, Åstot C, Blackwell J, Moritz T, Olsson O, Sandberg G (1997) Auxin-cytokinin interactions in wild-type and transgenic tobacco. Plant Cell Physiol 38:225–235
Emery RJN, Leport L, Barton JE, Turner NC, Atkins CA (1998) cis-Isomers of cytokinins predominate in chickpea seeds throughout their development. Plant Physiol 117:1515–1523
Emery RJN, Ma Q, Atkins CA (2000) The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol 123:1593–1604
Escalona VH, Aguayo E, Artés E (2007) Extending the shelf life of kohlrabi stems by modified atmosphere packaging. J Food Sci 72:308–313
Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Žižková E, Hanuš J, Dančák M, Trávníček B, Pešek B, Krupička M, Vaňková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 62:2827–2840
Ghnaya AB, Charles G, Branchard M (2008) Rapid shoot regeneration from thin cell layer explants excised from petioles and hypocotyls in four cultivars of Brassica napus L. Plant Cell Tissue Organ Cult 92:25–30
Glendening TM, Sjolund R (1988) In vitro propagation of kohlrabi from leaf explants. HortScience 23:772
Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM (2007) Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134:3539–3548
Grosch R, Schneider JHM, Kofoet A (2004) Characterisation of Rhizoctonia solani anastomosis groups causing bottom rot in field-grown lettuce in Germany. Eur J Plant Pathol 110:53–62
Haddadi P, Moieni A, Karimzadeh G, Abdollahi MR (2008) Effects of gibberellin, abscisic acid and embryo desiccation on normal plantlet regeneration, secondary embryogenesis and callogenesis in microspore culture of Brassica napus L. cv. PF704. Int J Plant Prod 2:153–162
Hansen LN, Ortiz R, Andersen SB (1999) Genetic analysis of protoplast regeneration ability in Brassica oleracea. Plant Cell Tissue Organ Cult 58:127–132
Jones B, Andersson Gunnerås S, Petersson SV, Tarkowski P, Graham N, May S, Dolezal K, Sandberg G, Ljung K (2010) Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22:2956–2969
Kamal GB, Illich KG, Asadollah A (2007) Effect of genotype, explant type and nutrient medium components on canola (Brassics napus L.) shoot in vitro organogenesis. Afr J Biotechnol 6:861–867
Kamínek M, Pačes V, Corse J, Challice JS (1979) Effect of stereospecific hydroxylation of N6-(∆2-isopentenyl)adenosine on cytokinin activity. Planta 145:239–243
Kamínek M, Motyka V, Vaňková R (1997) Regulation of cytokinin content in plant cells. Physiol Plant 101:689–700
Kamínek M, Březinová A, Gaudinová A, Motyka V, Vaňková R, Zažímalová E (2003) Purine cytokinins: a proposal of abbreviations. Plant Growth Regul 32:253–256
Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H (2004) Distinct isoprenoid origins of cis- and trans-zeatin biosyntheses in Arabidopsis. J Biol Chem 279:14049–14054
Khan MR, Rashid H, Ansar M, Chaudry Z (2003) High frequency shoot regeneration and Agrobacterium-mediated DNA transfer in Canola (Brassica napus). Plant Cell Tissue Organ Cult 75:223–231
Klemš M, Slámová Z, Motyka V, Malbeck J, Trávníčková A, Macháčková I, Holík J, Procházka S (2011) Changes in cytokinin levels and metabolism in tobacco (Nicotiana tabacum L.) explants during in vitro shoot organogenesis induced by trans-zeatin and dihydrozeatin. Plant Growth Regul 65:427–437
Klíma M, Vyvadilová M, Kučera V (2004) Production and utilization of doubled haploids in Brassica oleracea vegetables. Hortic Sci Prague 31:119–123
Koh WL, Loh CS (2000) Direct somatic embryogenesis, plant regeneration and in vitro flowering in rapid-cycling Brassica napus. Plant Cell Rep 19:1177–1183
Lexa M, Genkov T, Malbeck J, Macháčková I, Brzobohatý B (2003) Dynamics of endogenous cytokinin pools in tobacco seedlings: a modelling approach. Ann Bot 91:585–597
Lillo C, Shanin EA (1986) Rapid regeneration of plants from hypocotyl protoplasts and root segments of cabbage. HortScience 21:315–317
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Liu J, Mehdi S, Topping J, Tarkowski P, Lindsey K (2010) Modelling and experimental analysis of hormonal crosstalk in Arabidopsis. Mol Syst Biol 6: Article number 373
LoSchiavo F, Pitto L, Giuliano G, Torti G, Nuti-Ronchi V, Marazziti D, Vergara R, Orselli S, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Martin RC, Mok MC, Habben JE, Mok DWS (2001) A cytokinin gene from maize encoding an O-glucosyltransferase specific to cis-zeatin. Proc Natl Acad Sci USA 98:5922–5926
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37:128–138
Moghaieb R, El-Awady M, El Mergawy R, Youssef S, El-Sharkawy A (2006) A reproducible protocol for regeneration and transformation in canola (Brassica napus L.). Afr J Biotechnol 5:143–148
Mollika SR, Sarker RH, Hoque MI (2011) In vitro plant regeneration in Brassica spp. Plant Tissue Cult Biotechnol 21:127–134
Moncaleán P, Rodríguez A, Fernández B (2003) Effect of different benzyladenine time pulses on the endogenous levels of cytokinins, indole-3-acetic acid and abscisic acid in micropropagated explants of Actinidia deliciosa. Plant Physiol Biochem 41:149–155
Montalbán IA, Novák O, Rolčik J, Strnad M, Moncaleán P (2013) Endogenous cytokinin and auxin profiles during in vitro organogenesis from vegetative buds of Pinus radiata adult trees. Physiol Plant 148:214–231
Motte H, Vereecke D, Geelen D, Werbrouck S (2014) The molecular path to in vitro shoot regeneration. Biotechnol Adv 32:107–121
Motyka V, Vaňková R, Čapková V, Petrášek J, Kamínek M, Schmülling T (2003) Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiol Plant 117:11–21
Msikita W, Skirvin RM (1989) In vitro regeneration from hypocotyl and seedling cotyledons of tronchuda (Brassica oleracea var. tronchuda Bailey). Plant Cell Tissue Organ Cult 19:159–165
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
National Institute for Health and Welfare, Nutrition Unit. Fineli (2011) Finnish Food Composition Database Release 14, Helsinki 2011. http://www.fineli.fi/food.php?foodid=329&lang=en. Accessed 8 June 2012
Neelakandan AK, Wang K (2012) Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep 31:597–620
Nikolić R, Mitić N, Miletić R, Nešković M (2006) Effects of cytokinins on in vitro seed germination and early seedling morphogenesis in Lotus corniculatus L. J Plant Growth Regul 25:187–194
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101:8039–8044
Okubo H, Wada K, Uemoto S (1991) In vitro morphogenetic response and distribution of endogenous plant hormones in hypocotyl segment of snapdragon (Antirrhinum majus L.). Plant Cell Rep 10:501–504
Ono Y, Takahata Y, Kaizuma N (1994) Effect of genotype on shoot regeneration from cotyledonary explants of rapeseed (Brassica napus L.). Plant Cell Rep 14:13–17
Otto F (1988) High resolution DNA—flow cytometry using DAPI, Protocol 1, Partec Arlesheim, Münster
Ovesná J, Ptacek L, Opatrny Z (1993) Factors influencing the regeneration capacity of oilseed rape and cauliflower in transformation experiments. Biol Plant 35:107–112
Parker CW, Entsch B, Letham DS (1986) Inhibitors of two enzymes which metabolize cytokinins. Phytochemistry 25:303–310
Paul S, Sikdar SR (2005) Regeneration of plants from root explant of two Indian cultivars of Brassica campestris L. through somatic embryogenesis. Curr Sci 89:1323–1326
Pavlović S, Vinterhalter B, Mitić N, Adžić S, Pavlović N, Zdravković M, Vinterhalter D (2010) In vitro shoot regeneration from seedling explants in Brassica vegetables: red cabbage, broccoli, Savoy cabbage and cauliflower. Arch Biol Sci 62:337–345
Pellegrineschi A (1997) In vitro plant regeneration via organogenesis of cowpea [Vigna unguiculata (L.) Walp.]. Plant Cell Rep 17:89–95
Pernisová M, Klíma P, Horák J, Válková M, Malbeck J, Souček P, Reichman P, Hoyerová K, Dubová J, Friml J, Zažímalová E, Hejátko J (2009) Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc Natl Acad Sci USA 106:3609–3614
Raspor M, Motyka V, Žižková E, Dobrev PI, Trávníčková A, Zdravković-Korać S, Simonović A, Ninković S, Dragićević IC (2012) Cytokinin profiles of AtCKX2-overexpressing potato plants and the impact of altered cytokinin homeostasis on tuberization in vitro. J Plant Growth Regul 31:460–470
Ravanfar SA, Aziz MA, Kadir MA, Rashid AA, Haddadi F (2011) In vitro adventitious shoot regeneration and acclimatisation of Brassica oleracea subsp. italica cv Green Marvel. Afr J Biotechnol 10:5614–5619
Ravanfar SA, Aziz MA, Rashid AA, Salim S (2014) In vitro adventitious shoot regeneration from cotyledon explant of Brassica oleracea subsp. italica and Brassica oleracea subsp. capitata using TDZ and NAA. Pak J Bot 46:329–335
Schmitz RY, Skoog F, Playtis AJ, Leonard NJ (1972) Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiol 50:702–705
Selman LW, Kulasegaram S (1966) Development of the stem tuber in kohlrabi. J Exp Bot 18:471–490
Sharma KK, Thorpe TA (1989) In vitro regeneration of shoot buds and plantlets from seedling root segments of Brassica napus L. Plant Cell Tissue Organ Cult 18:129–141
Souza BM, Kraus JE, Enders L, Mercier H (2003) Relationships between endogenous hormonal levels and axillary bud development of Ananas comosus nodal segments. Plant Physiol Biochem 41:733–739
Sparrow PAC, Townsend TM, Morgan CL, Dale PJ, Arthur AE, Irwin JA (2004) Genetic analysis of in vitro shoot regeneration from cotyledonary petioles of Brassica oleracea. Theor Appl Genet 108:1249–1255
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45:1299–1305
Sretenović-Rajičić T, Ninković S, Vinterhalter B, Miljuš-Đukić J, Vinterhalter D (2004) Introduction of resistance to herbicide Basta® in Savoy cabagge. Biol Plant 48:431–436
Sretenović-Rajičić T, Ninković S, Miljuš-Đukić J, Vinterhalter B, Vinterhalter D (2006) Agrobacterium rhizogenes-mediated transformation of Brassica oleracea var. sabauda and B. oleracea var. capitata. Biol Plant 50:525–530
Stirk WA, Novák O, Václavíková K, Tarkowski P, Strnad M, van Staden J (2008) Spatial and temporal changes in endogenous cytokinins in developing pea roots. Planta 227:1279–1289
Stirk WA, Václavíková K, Novák O, Gajdošová S, Kotland O, Motyka V, Strnad M, van Staden J (2012) Involvement of cis-zeatin, dihydrozeatin, and aromatic cytokinins in germination and seedling establishment of maize, oats, and lucerne. J Plant Growth Regul 31:392–405
Suri SS, Saini ARK, Ramawat KG (2005) High frequency regeneration and Agrobacterium tumefaciens-mediated transformation of broccoli (Brassica oleracea var. italica). Eur J Hortic Sci 70:71–78
Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279:41866–41872
Teo W, Lakshmanan P, Kumar P, Goh CJ, Swarup S (1997) Direct shoot formation and plant regeneration from cotyledon explants of rapid-cycling Brassica rapa. In Vitro Cell Dev Biol Plant 33:288–292
Valdés AE, Ordas RJ, Fernandez B, Centeno ML (2001) Relationships between hormonal contents and the organogenic response in Pinus pinea cotyledons. Plant Physiol Biochem 39:377–384
Veach YK, Martin RC, Mok DWS, Malbeck J, Vaňková R, Mok MC (2003) O-Glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131:1374–1380
Verma SK, Yücesan BB, Şahin G, Gürel S, Gürel E (2011) Direct shoot regeneration from leaf explants of Digitalis lamarckii, an endemic medicinal species. Turk J Bot 35:689–695
Vinterhalter D, Sretenović-Rajičić T, Vinterhalter B, Ninković S (2007) Genetic transformation of Brassica oleracea vegetables. Transgenic Plant J 1:340–355
Wang Y, Tong Y, Li Y, Zhang Y, Zhang J, Feng J, Feng H (2011) High frequency plant regeneration from microspore-derived embryos of ornamental kale (Brassica oleracea L. var. acephala). Sci Hortic 130:296–302
Wong KW, Loh CS (1988) In vitro regeneration of plantlets from root segments of Brassica alboglabra Bailey. Sci Hortic Amst 35:7–14
Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H (2004) Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol 134:1654–1661
Zhang Y, Xu J, Han L, Wei W, Guan Z, Cong L, Chai T (2006) Efficient regeneration and Agrobacterium-mediated transformation of Brassica juncea. Plant Mol Biol Rep 24:255a–255i
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of Serbia (No. 173015) and the Czech Science Foundation (P506/11/0774).
Author information
Authors and Affiliations
Corresponding author
Additional information
Tatjana Ċosiċ and Václav Motyka have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ċosiċ, T., Motyka, V., Raspor, M. et al. In vitro shoot organogenesis and comparative analysis of endogenous phytohormones in kohlrabi (Brassica oleracea var. gongylodes): effects of genotype, explant type and applied cytokinins. Plant Cell Tiss Organ Cult 121, 741–760 (2015). https://doi.org/10.1007/s11240-015-0742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0742-2