Abstract
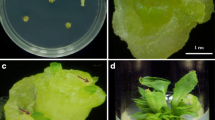
Reproducible and highly efficient protocols for shoot regeneration and genetic transformation mediated by Agrobacterium have been established for safflower (Carthamus tinctorius L.). Agrobacterium tumefaciens strain LBA 4404 with gus reporter gene and hygromycin (hpt gene) as plant selection marker was used as the plant transformation vector. Genetic transformation experiments were carried out to evaluate the efficacy of various parameters such as genotype, seedling age, co-cultivation period, bacterial titer, enzymatic pre-treatment of target tissues, use of compounds that induce vir-gene enhancer, acetosyringone (AS), explant type and explant injury to enhance transformation efficiency. Transformation frequency was high when root and hypocotyl explants of 8-day-old seedlings of safflower cv. HUS-305 were co-cultivated with bacterial cell density of 0.5 OD600 during a period of 2 days followed by selection regime of 10–15–15 mg/l hygromycin. The frequency of rooting of the primary transformants was low (18.0%) when compared with the regenerated shoots (70.0%), and seven shoots survived on transfer to soil. The putative transformants were confirmed by β-glucuronidase (GUS) histochemical assay, polymerase chain reaction (PCR), reverse-transcriptase PCR (RT-PCR) and Southern blot analysis. With the optimized transformation protocol, putative transformed shoots were obtained with frequency of 51.0% within 8–10 weeks of culture initiation.




Similar content being viewed by others
Abbreviations
- 2,4,5-Cl3POP:
-
2,4,5-Trichlorophenoxypropionic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- 2iP:
-
N6-[2-Isopentenyl]adenine
- AS:
-
Acetosyringone
- AgNO3 :
-
Silver nitrate
- BA:
-
6-Benzyladenine
- GUS:
-
β-Glucuronidase
- hpt :
-
Hygromycin phosphotransferase gene
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- KN:
-
6-Furfurylaminopurine/kinetin
- MS:
-
Murashige and Skoog medium
- NAA:
-
α-Naphthaleneacetic acid
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse-transcriptase polymerase chain reaction
- PGA:
-
Phloroglucinol
- TDZ:
-
Thidiazuron
- X-GlcA:
-
5-Bromo-4-chloro-3-indolyl-β-d-glucuronic acid
References
Agnieszka G, Malgorzata K, Edward Z (2004) Conditions of transformation and regeneration of ‘Induka’ and ‘Elista’ strawberry plants. Plant Cell Tiss Org Cult 79:153–160. doi:10.1007/s11240-004-0655-y
Bond JE, Roose ML (1998) Agrobacterium-mediated transformation of the commercially important citrus cultivar Washington navel orange. Plant Cell Rep 18:229–234. doi:10.1007/s002990050562
Cervera M, Pina JA, Juarez J, Navarro L, Pena L (1998) Agrobacterium-mediated transformation of citrange: factors affecting transformation and regeneration. Plant Cell Rep 18:271–278. doi:10.1007/s002990050570
Dajue L, Mundel HH (1996) Saffower. Carthamus tinctorius L. promoting the conservation and use of underutilized and neglected crops, vol 7. Institute of Plant Genetics and Crop Plant Research, Gaterslaben/International Plant Genetic Resources Institute, Rome, Italy 83 pp
Doyle J, Doyle J (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Economou A, Read PE (1981) Improving the efficiency of Petunia propagation from leaf segments cultured in vitro. Hort Sci 16:406
Fiorino P, Leva AR (1983) Propagation of apple cultivars. Acta Hort 131:95–99
George L, Rao PS (1982) In vitro multiplication of safflower (Carthamus tinctorius L.) through tissue culture. Proc Indian Natl Sci Acad B 48:791–794
Gürel E, Kazan K (1999) Evaluation of various sunflower (Helianthus annuus L.) genotypes for Agrobacterium tumefaciens-mediated gene transfer. Turkish J Bot 23:171–177
Gutierrez-E MA, Luth D, Moore GA (1997) Factors affecting Agrobacterium-mediated transformation in citrus and production of sour orange (Citrus aurantium L.) plants expressing the coat protein gene of Citrus tristeza virus. Plant Cell Rep 16:745–753. doi:10.1007/s002990050313
Holford P, Hernandez N, Newburg HT (1992) Factors influencing the efficiency of T-DNA transfer during co-cultivation ofAntirrhinum majus with Agrobacterium tumefaciens. Plant Cell Rep 11:196–199. doi:10.1007/BF00232532
Jefferson RA (1987) Assaying chimeric genes in plants, the GUS gene fusion system. Plant Mol Biol Rep 5:387–405. doi:10.1007/BF02667740
Jeyaramraja PR, Nithya (2005) Agrobacterium tumefaciens-mediated transformation of embryogenic tissues of tea (Camellia sinensis (L.) O. Kuntze). Plant Mol Biol Rep 23:299a–299i. doi:10.1007/BF02772761
Jong HK, Botella JR (2002) Callus induction and plant regeneration from broccoli (Brassica oleracea var. italica) for transformation. J Plant Biol 45:177–181. doi:10.1007/BF03030311
Lane WD, McDougald JM (1982) Shoot tip culture of apple: comparative response of five cultivars to cytokinin and auxin. Canad J Plant Sci 62:689–694
Li HY, Zhu YM, Chen Q, Conner RL, Ding XD, Li J, Zhang BB (2004) Production of transgenic fertile plants by direct somatic embryogenesis from immature zygotic embryos of transgenic soybean plants with two anti-fungal protein genes via Agrobacterium and particle bombardment. Biol Plant 48:367–374. doi:10.1023/B:BIOP.0000041088.62614.76
Mandal AKA, Gupta SD (2001) Direct shoot organogenesis and plant regeneration in safflower. In Vitro Cell Dev Biol Plant 37:50–54. doi:10.1007/s11627-001-0010-5
Mandal AKA, Gupta SD (2003) Somatic embryogenesis of safflower: influence of auxin and ontogeny of somatic embryos. Plant Cell Tiss Org Cult 72:27–31. doi:10.1023/A:1021264403398
Mandal AKA, Chatterjee AK, Gupta SD (1995) Direct somatic embryogenesis and plantlet regeneration from cotyledonary leaves of safflower. Plant Cell Tiss Org Cult 43:287–289. doi:10.1007/BF00039957
Mandal AKA, Chatterjee AK, Gupta SD (2001) Factors affecting somatic embryogenesis from cotyledonary explants of safflower. Biol Plant 44:503–507. doi:10.1023/A:1013722116224
Mohamed SH, Boehm R, Binsfeld PC, Schnabl H (2004) Agrobacterium-mediated transformation of two high oleic sunflower (Helianthus annuus L.) genotypes: assessment and optimization of important parameters. Helia 27:25–40
Mukhopadhyay A, Arumugam N, Nandakumar PBA, Pradhan AK, Gupta V, Pental D (1992) Agrobacterium-mediated genetic transformation of oilseed Brassica campestris: Transformation frequency is strongly influenced by the mode of shoot regeneration. Plant Cell Rep 11:506–513. doi:10.1007/BF00236266
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobaccco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Neetika W, Amandeep K, Shashi BB (2005) In vitro regeneration of a high oil-yielding variety of safflower (Carthamus tinctorius var HUS-305). J Plant Biochem Biotech 14:65–68
Nikam TD, Shitole MG (1999) In vitro culture of safflower L. cv. Bhima: initiation, growth, optimization and organogenesis. Plant Cell Tiss Org Cult 55:15–22. doi:10.1023/A:1026493616991
Öktem HA, Bülbül Y, Öktem E, Yücel M (1999) Regeneration and Agrobacterium-mediated transformation studies in tomato (Lycopersicon esculentum Miller). Turkish J Bot 23:345–348
Orlikowska TK, Dyer WE (1993) In vitro regeneration and multiplication of safflower (Carthamus tinctorius L.). Plant Sci 93:151–157. doi:10.1016/0168-9452(93)90044-Z
Orlikowska TK, Cranston JH, Dyer WE (1995) Factors influencing Agrobacterium tumefaciens mediated transformation of the safflower cv. ‘Centennial’. Plant Cell Tiss Org Cult 40:85–91. doi:10.1007/BF00041122
Radhika K, Sujatha M, Rao TN (2006) Thidiazuron stimulates adventitious shoot regeneration in different safflower explants. Biol Plant 50:174–179. doi:10.1007/s10535-006-0003-7
Rao SK, Rohini VK (1999) Gene transfer into Indian cultivars of safflower (Carthamus tinctorius L.) using Agrobacterium tumefaciens. Plant Biotech 16:201–206
Rohini VK, Rao SK (2000) Embryo transformation. A practical approach for realizing transgenic plants of safflower (Carthamus tinctorius L.). Ann Bot 86:1043–1049. doi:10.1006/anbo.2000.1278
Rubén A, Ricardo JO (2007) Improved genetic transformation protocol for cork oak (Quercus suber L.). Plant Cell Tiss Org Cult 91:45–52. doi:10.1007/s11240-007-9276-6
Saima T, Bushra M (2004) Factors affecting Agrobacterium tumefaciens-mediated genetic transformation of Vigna radiata (L.) Wilczek. Pak J Bot 36(4):887–896
Saini R, Jaiwal PK (2007) Agrobacterium tumefaciens-mediated transformation of blackgram: an assessment of factors influencing the efficiency of uidA gene transfer. Biol Plant 51:69–74. doi:10.1007/s10535-007-0014-z
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanatombi K, Sharma GJ (2008) In vitro plant regeneration in six cultivars of Capsicum spp. using different explants. Biol Plant 52:141–145. doi:10.1007/s10535-008-0029-0
Sawahel W, Hagran A (2006) Generation of white mold disease-resistant sunflower plants expressing human lysozyme gene. Biol Plant 50:683–687. doi:10.1007/s10535-006-0106-1
Sheng JL, Zhi-Ming W, Jian-Qiu H (2008) The effect of co-cultivation and selection parameters on Agrobacterium-mediated transformation of Chinese soybean varieties. Plant Cell Rep 27:489–498. doi:10.1007/s00299-007-0475-8
Sridevy S, Heiko M, Margrethe S (2008) Regeneration and transformation in adult plants of Campanula species. Plant Cell Rep 27:1713–1720. doi:10.1007/s00299-008-0590-1
Srivastava T, Sandip D, Sudhir Kumar S, Srivastava PS (2009) A reliable protocol for transformation of Catharanthus roseus through Agrobacterium tumefaciens. Physiol Mol Biol Plants 15:93–98. doi:10.1007/s12298-009-0010-1
Sujatha M, Suganya A (1996) In vitro organogenic comparison of different seedling tissues of safflower (Carthamus tinctorius L.). Sesame Safflower Newsl 11:85–90
Sunil Kumar G, Keerti SR (2001) Transgenic cotton: factors influencing Agrobacterium- mediated transformation and regeneration. Mol Breed 8:37–52. doi:10.1023/A:1011906701925
Uranbey S, Sevimay CS, Kaya MD, Ipek A, Sancak C, Basalma D, Er C, Ozcan S (2005) Influence of different cocultivation temperatures, periods and media on Agrobacterium tumefaciens-mediated gene transfer. Biol Plant 49:53–57. doi:10.1007/s10535-005-3057-z
Vijaya Kumar J, Ranjitha Kumari BD, Enrique C (2008) Cyclic somatic embryogenesis and efficient plant regeneration from callus of safflower. Biol Plant 52:429–436. doi:10.1007/s10535-008-0087-3
Walia N, Kaur A, Babbar SB (2007) Proliferation and differentiation from endosperms of Carthamus tinctorius. Biol Plant 51:749–753. doi:10.1007/s10535-007-0153-2
Wang HM, Zu YG (2007) Agrobacterium-mediated genetic transformation of Camptotheca acuminate. J For Res 18:316–318. doi:10.1007/s11676-007-0064-2
Ying M, Dyer WT, Bergman J (1992) Agrobacterium tumefaciens-mediated transformation of safflower (Carthamus tinctorius L.) cv. ‘Centennial’. Plant Cell Rep 11:581–585. doi:10.1007/BF00233097
Zhao HM, Liang AH, Yang WC (2007) Effects of hygromycin on cotton cultures and its application in Agrobacterium-mediated cotton transformation. In Vitro Cell Dev Biol Plant 43:111–118. doi:11810.1007/s11627-007-9031-z
Acknowledgments
We thank Dr. D. M. Hegde, Project Director, Directorate of Oilseeds Research, Hyderabad, India for extending all the research facilities for carrying out the study. The guidance and support of Dr. S. M. Balachandran, Directorate of Rice Research, Hyderabad, India in carrying out Southern blot hybridization work is acknowledged. The first author is grateful to Jawaharlal Nehru Memorial Fund, New Delhi, India for providing a fellowship for the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sri Shilpa, K., Dinesh Kumar, V. & Sujatha, M. Agrobacterium-mediated genetic transformation of safflower (Carthamus tinctorius L.). Plant Cell Tiss Organ Cult 103, 387–401 (2010). https://doi.org/10.1007/s11240-010-9792-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9792-7