Abstract
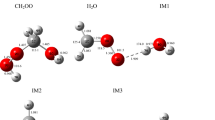
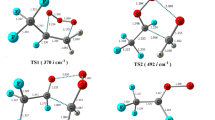
The reaction of C2H5OH and O3 on the singlet potential energy surface is carried out using the MP2 and CCSD(T)//MP2 theoretical approaches in connection with the 6-311++G(d,p) basis set. Three pre-reactive complexes C1, C2, and C3 are formed between ethanol and ozone at atmospheric pressure and 298.15 K temperature. With variety of the complexes, seven types of product are obtained which four types of them have enough thermodynamic stability. In thermodynamic approach, the most favor product begins with the formation of pre-reactive C2 complex and produces the CH3CH(OH)2 + O2 as final adduct in a process that is computed to be exothermic by −53.759 kcal/mol and spontaneous reaction by −51.833 kcal/mol in Gibbs free energy. In kinetic viewpoint, the formation of CH3COH + cis-H2O3 as a final adducts is the most favor path.



Similar content being viewed by others
References
Goodarzi M, Vahedpour M, Solimannejad M (2012) Struct Chem 23:381
Vahedpour M, Zolfaghari F (2011) Struct Chem 22:1331
Vahedpour M, Goodarzi M, Hajari N, Nazari F (2011) Struct Chem 22:817
Siegbahn PEM (2003) Q Rev Biophys 36:91
Farrokhpour H, Fathi F (2011) J Comput Chem 32:2479
Lin Y, Tanaka S (2006) Appl Microbiol Biotechnol 69:627
Energy Information Administration, Alternatives to Traditional Transportation Fuels (1996) DOE/EIA-0585
Badger PC (2002) Ethanol from cellulose: a general review. In: Janick J, Whipkey A (eds) Trends in new crops and new uses. American Society for Horticultural Science (ASHS) Press, Alexandria, VA
Li J, Kazakov A, Dryer LF (2005) In: Proceedings of the European Combustion Meeting. http://www.princeton.edu/~combust
Morais PB, Rosa CA, Linardi VR, Carazza F, Nonato EA (1996) Biotechnol Lett 18:1351
Li J, Kazakov A, Chaos M, and Drye FL, (2007) In: 5th US Combustion Meeting Organized by the Western States Section of the Combustion Institute and Hosted by the University of California at San Diego, March 25–28
Marinov NM (1999) Int J Chem Kinet 31:183
Shereshovets VV, Shafikov NY, Lomakin GS, Ivanov AI, Akhunov IR, Ponomarev OA, Komissarov VD (1985) Russ Chem Bull 34:1157
Oyama ST, Li W (1997) Stud Surf Sci Catal 110:873
Oyama ST, Li W, Zhang W (1999) Stud Surf Sci Catal 121:105
Mayotte S, Lindhjem C, Rao V, Sklar M (1994) Reformulated gasoline effects on exhaust emissions: phase I: initial investigation of oxygenate, volatility, distillation and sulfur effects. SAE Paper No. 941973
Carr SA, Blitz MA, Seakins PW (2011) J Phys Chem A 115:3335
Fournet R, Glaude PA, Bounaceu1 R, Molière M (2009) In: Proceeding of the European Combustion Meeting
Carvalho EFV, Barauna AN, Machado FBC, Roberto-Neto O (2008) Int J Quantum Chem 108:2476
Clary DC, Kerkeni B (2006) Phys Chem Chem Phys 8:917
Kugel R, Aube HT (1975) J Phys Chem 79:2130
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265
Cizek J (1969) Adv Chem Phys 14:35
Lee TJ, Taylor PR (1989) Int J Quantum Chem Symp 23:199
Jayatilaka D, Lee TJ (1993) J Chem Phys 98:9734
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 (Revision B 03). Gaussian, Inc., Pittsburgh, PA
Butkovskaya NL, Zhao Y, Setzer DW (1994) J Phys Chem 98:10779
Alecu IM, Truhlar DG (2011) J Phys Chem A 115:2811
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Bader RFW (1991) Chem Rev 91:893
Carvalho EFV, Barauna AN, Machado FBC, Roberto-Neto O (2008) Int J Quantum Chem 108:2476
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shayan, K., Vahedpour, M. Computational study on the reaction mechanism of atmospheric oxidation of ethanol with ozone. Struct Chem 24, 611–621 (2013). https://doi.org/10.1007/s11224-012-0111-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0111-2