Abstract
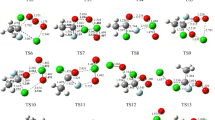
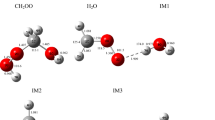
Reaction pathways of methylamine with ozone on the singlet potential energy profile have been investigated at the RB3LYP/6-311 ++G (3df–3pd) computational level. Calculated results reveal that six kinds of products P1 (CH3NO + H2O2), P2 (CH3NH + OH + O2), P3 (NH2CH + HO2+ OH), P4 (CH2NH + H2O +O2), P5 (NH2CH2OH + O2), P6 (NH3+ CH2O +O2) are obtained through variety of transformation of one reactant complex C1. Cleavage and formation of the chemical bonds in the reaction pathways have been discussed using the structural parameters. Based on the calculations, the title reaction leads to NH3+ CH2O + O2 as thermodynamic adducts in an exothermic process by −76.28 kcal/mol in heat realizing and spontaneous reaction by −86.71 kcal/mol in standard Gibbs free energy. From a kinetic viewpoint, the production of CH3NH + OH + O2 adducts with one transition state is the most favoured path.

A possible mechanism for the CH3NH2+O3 reaction on the singlet state are suggested. Six kinds of products from one reactant complex C1 are obtained. Cleavage and formation of chemical bonds in the reaction pathways are discussed using structural parameters.




Similar content being viewed by others
References
Mitchell S C and Zhang A Q 2001 Clin. Chim. Acta 312 107
Corbin D, Schwarz S and Sonnichsen G 1997 Catal. Today 37 71
Huerta F, Morallon E, Perez J M, Vazquez J L and Aldaz A 1999 J. Electroanal. Chem. 469 159
Tian W, Wang W, Zhang Y and Wang W 2009 Int. J. Quantum. Chem. 109 1566
Liua J, Lva C, Guoa Y and Wangb G 2013 Appl. Surf. Sci. 271 291
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid$=$3518#x351
Li X, Meng L and Zhang S 2007 J. Mol. Struct. 847 52
Alhambra C, Sanchez M L, Corchado J C, Gao J and Truhlar D G 2002 Chem. Phys. Lett. 355 388
Kaye J A and Strobel D F 1983 ICARUS 55 399
Zhu S, Li Q, Dua Y, Yang X, Fan J and Dong Z 2010 Toxicol. In Vitro 24 809
Chintharlapalli S, Papineni S, Baek S J, Liu S and Safe S 2005 Mol. Pharmacol. 68 1782
Peel J B and Willett G D 1975 J. Chem. Soc. Faraday Trans. II 71 1799
Tiwary S and Mukherjee A 2009 J. Mol. Struct.: THEOCHEM 3 57
Zhang L, Liu H, Tang H and Huang T 2014 Chem. Pap. 68 145
Kayi H, Kaiser R and Head J 2011 Phys. Chem. Chem. Phys. 13 11083
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Oritz J V, Cui Q, Baboul A G, Clifford S, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A 2003 Gaussian 03, Revision B.03. Gaussian Inc., Pittsburgh, PA
Becke A D (993 J. Chem. Phys. 98 1372
Lee C, Yang W and Parr R G 1988 Phys. Rev. B37 785
Biegler-Kning F 2000 AIM2000 Ver 1.0. University of Applied Science, Bielefeld, Germany
Bader R F W 1990 Atoms in molecules – a quantum theory (Oxford: Oxford University Press)
Bader R F W 1991 Chem. Rev. 91 893
NIST Chemistry WebBook, NIST Standard Reference Database Number 69, www.nist.gov
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Cartesian coordinate and frequencies of the reactants, products, intermediates and transition states involved in the CH3NH2+ O3reaction at the RB3LYP/6-311 + G(3df-3pd) level of theory are collected in the supplementary data. For details, see www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
VALEHI, S., VAHEDPOUR, M. Theoretical study on the mechanism of CH 3 NH 2 and O 3 atmospheric reaction. J Chem Sci 126, 1173–1180 (2014). https://doi.org/10.1007/s12039-014-0640-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0640-x