Abstract
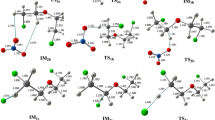
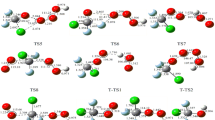
The reaction mechanism of SO2 with O3 on the singlet potential energy surface has been investigated theoretically at the G3MP2B3//B3LYP/6-311+G(3df) level of theory. The reactants are initially associated with adducts IN1(O2S–OOO) and IN2(OS-cyclic O4) in a barrier-less process. Subsequently, these adducts undergo isomerization and dissociation processes to produce cis-OSOO + 3O2, SO3(C s ) + 3O2 and SO3(D 3h ) + 3O2 products. The SO3(D 3h ) + 3O2 is major product and the cis-OSOO + 3O2 and SO3(C s ) + 3O2 are minor products. No stable pathway has been found for the formation of trans-OSOO and cyclic-SOOO isomers in the reaction of SO2 + O3. For major product, the rate constant of SO2 + O3 reaction is 2.30 × 10−23 cm3 molecule−1 s−1, at room temperature and atmospheric pressure.



Similar content being viewed by others
References
Farquhar J, Bao H, Thiemens M (2000) Science 289:756
Habicht KS, Gade M, Thamdrup B, Berg P, Canfield DE (2002) Science 298:2372
Ballester MY, Caridade PJSB, Varandas AJC (2007) Chem Phys Lett 439:301
Jou S, Shen M, Yu C, Lee Y (1996) J Chem Phys 104:15
Wayne RP (1991) The chemistry of the atmosphere, 2nd edn. Clarendon, Oxford
Calvert JC, Lazarus A, Kok GL, Heikes BG, Walega JG, Lind J, Cantrell CA (1985) Nature 317:27
Leopold KR, Bowen KH, Klemperer W (1981) J Chem Phys 74:4211
Bent R, Ladner WR (1963) Spectrochim Acta 19:931
Dorney AJ, Hoy AR, Mills IM (1973) J Mol Spectrosc 45:253
Tang SY, Brown CW (1975) J Raman Spectrosc 3:387
Kaldor A, Maki AG, Dorney AJ, Mills IM (1973) J Mol Spectrosc 45:247
Brassington NJ, Edwards HGM, Farwell DW, Long DA, Mansour HR (1978) J Raman Spectrosc 7:154
Henfrey NF, Thrush BA (1983) Chem Phys Lett 102:135
Bondybey VE, English JH (1985) J Mol Spectrosc 109:221
March J (1985) Advanced organic chemistry, 3rd edn. Wiley, New York
Atkinson R, Cater WPL (1984) Chem Rev 84:437
Atkinson R (1990) Atoms Environ 24A:1
Atkinson R, Aschmann SM (1993) Environ Sci Technol 27:1357
Baird C (1998) Environmental chemistry. Freeman, New York
Goodarzi M, Vahedpour M, Nazari F (2010) Struct Chem 21:915
Xu ZF, Lin MC (2007) Chem Phys Lett 440:12
Peiró-García J, Nebot-Gil I (2004) Chem Phys Lett 391:195
De Petris G, Cartoni A, Rosi M, Troiani A (2005) Chem Phys Lett 410:377
Varandas AJC, Zhang L (2004) Chem Phys Lett 385:409
Goodarzi M, Piri F, Hajari N, Karimi L (2010) Chem Phys Lett 499:51
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 (Revision B 03). Gaussian, Inc, Pittsburgh, PA
Goodarzi M, Vahedpour M, Nazari F (2010) Chem Phys Lett 494:315
Goodarzi M, Vahedpour M, Nazari F (2010) Chem Phys Lett 497:1
Becke AD (1993) J Chem Phys 98:1372
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JAJ (1998) Chem Phys 109:7764
Boboul AG, Curtiss LA, Redfern PC, Raghavachari KJ (1999) Chem Phys 110:7650
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Schofield K (2001) Combust Flame 124:137
Hills A, Cicerone R, Calvert J, Birks J (1987) J Phys Chem 91:1199
Eyring H (1935) J Chem Phys 3:1079
Evans MG, Polanyi M (1935) Trans Faraday Soc 31:875
Miyoshi A (2010) Gaussian post processor (GPOP). University of Tokyo, Tokyo
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vahedpour, M., Goodarzi, M., Hajari, N. et al. Theoretical study on the atmospheric formation of sulfur trioxide as the primary agent for acid rain. Struct Chem 22, 817–822 (2011). https://doi.org/10.1007/s11224-011-9758-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9758-3