Abstract
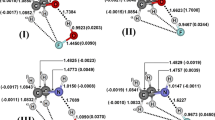
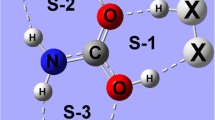
B3LYP/6-311++G(d,p) calculations were employed in order to examine the molecular parameters of the C2H3XS···NH3 heterocyclic hydrogen-bonded complexes with X = H, F and CH3. Intermolecular criteria were taken into account when studying the formation of these hydrogen-bonded complexes, such as geometry analysis, charge density quantification and interpretation of the harmonic vibrational spectrum, in which case the appearance of red-shift and blue-shift effects was discussed. It was assumed from the outset that many hydrogen bond types may exist in these systems, and these were investigated using the results of topological integrations from the quantum theory of atoms in molecules (QTAIM) and intermolecular charge transfer calculations using the ChelpG scheme. The proton donor/acceptor behavior of C2H3XS was interpreted in terms of hydrogen bond energies, whose values were corrected using the basis sets superposition error (BSSE) and zero point energy (ZPE).



Similar content being viewed by others
References
Burnett JF, Zahler RE (1951) Chem Rev 49:273–412. doi:10.1021/cr60153a002
Bobylev VA, Koldobskii SG, Tereshchenko GF, Gidaspov BV (1988) Chem Heter Comp 24:947–959. doi:10.1007/BF00474036
Skancke A, Van Vechten D, Liebman JF, Skancke PN (1996) J Mol Struct 376:461–468. doi:10.1016/0022-2860(95)09062-2
Fokin AV, Kolomiets AF, Rudnitskaya LS, Shevchenko VI (1975) Russ Chem Bull 24:582–584. doi:10.1007/BF00927483
Siri D, Gaudel-Siria A, Pons J-M, Liotard D, Rajzmann M (2002) J Mol Struct (THEOCHEM) 588:71–78. doi:10.1016/S0166-1280(02)00133-1
Banks HD (2003) J Org Chem 68:2639–2644. doi:10.1021/jo0268411
Oliveira BG, Santos ECS, Duarte EM, Araújo RCMU, Ramos MN, Carvalho AB (2004) Spectrochim Acta [A] 60:1883–1887. doi:10.1016/j.saa.2003.10.006
Oliveira BG, Duarte EM, Araújo RCMU, Ramos MN, Carvalho AB (2005) Spectrochim Acta [A] 61:491–494. doi:10.1016/j.saa.2004.04.023
Cosléou J, Lister DG, Legon AC (1994) Chem Phys Lett 231:151–158. doi:10.1016/0009-2614(95)90571-5
Banks HD, White WE (2001) J Org Chem 66:5981–5986. doi:10.1021/jo001719s
Parker RE, Isaacs NS (1959) Chem Rev 59:737–799. doi:10.1021/cr50028a006
Holubka JW, Bach RD, Andrés JL (1992) Macromolecules 25:1189–1192. doi:10.1021/ma00029a028
Hohenberg P, Kohn W (1964) Phys Rev B 136:864–871. doi:10.1103/PhysRev.136.B864
Oliveira BG, Araújo RCMU, Ramos MN (2008) J Mol Model 14:949–955. doi:10.1007/s00894-008-0337-5
Bader FRW (1990) Atoms in molecules. A quantum theory. Oxford University Press, Oxford
Hati S, Datta D (1992) J Comput Chem 13:912–918. doi:10.1002/jcc.540130716
Bone RGA, Bader RFW (1996) J Phys Chem 100:10892–10911. doi:10.1021/jp953512m
Alkorta I, Rozas I, Elguero J (1998) Struct Chem 9:243–247. doi:10.1023/A:1022424228462
Legon AC (1995) Chem Phys Lett 247:24–31. doi:10.1016/0009-2614(95)01172-9
Oliveira BG, Araújo RCMU, Carvalho AB, Lima EF, Silva WLV, Ramos MN, Tavares AM (2006) J Mol Struct (THEOCHEM) 775:39–45. doi:10.1016/j.theochem.2006.06.028
Oliveira BG, Araújo RCMU, Carvalho AB, Ramos MN (2007) J Theor Comput Chem 6:647–660. doi:10.1142/S0219633607003362
Oliveira BG, Vasconcellos MLAA (2007) J Mol Struct (THEOCHEM) 774:83–88. doi:10.1016/j.theochem.2006.06.018
Wiberg KB, Breneman CM (1990) J Am Chem Soc 112:8765–8775. doi:10.1021/ja00180a019
Gillespie RJ (1978) Molecular geometry. Van Nostrand-Reinhold, London
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA, Gaussian 98 W (Revision A.11.2), Gaussian, Inc., Pittsburgh PA, 2001
Kolboe S, Svelle S (2008) J Phys Chem A 112:6399–6400. doi:10.1021/jp8027879
AIM 2000 1.0 designed by Biegler-König F, University of Applied Sciences, Bielefeld, Germany
van Duijneveldt FB, Murrell JN (1967) J Chem Phys 46:1759–1767. doi:10.1063/1.1840932
Boys SB, Bernardi F (1970) Mol Phys 19:553–566. doi:10.1080/00268977000101561
Oliveira BG, Araújo RCMU, Ramos MN (2008) Struct Chem 19:185–189. doi:10.1007/s11224-007-9269-4
Oliveira BG, Araújo RCMU, Ramos MN (2008) Struct Chem 19:665–670. doi:10.1007/s11224-008-9344-5
Grabowski SJ (2007) J Phys Chem A 111:3387–3393. doi:10.1021/jp070530i
Grabowski SJ, Sokalski WA, Leszczynski J (2006) Chem Phys Lett 432:33–39. doi:10.1016/j.cplett.2006.10.069
Oliveira BG, Pereira FS, Araújo RCMU, Ramos MN (2006) Chem Phys Lett 427:181–184. doi:10.1016/j.cplett.2006.06.019
Araújo RCMU, Ramos MN (1996) J Mol Struct (THEOCHEM) 366:233–240. doi:10.1016/0166-1280(96)04536-8
Pauling L (1960) The nature of the chemical bond, 3 edn. Cornell University, USA
Oliveira BG, Araújo RCMU, Carvalho AB, Ramos MN (2007) J Theor Comput Chem 6:647–660
Oliveira BG, Araújo RCMU, Carvalho AB, Ramos MN (2009) J Mol Model 15:123–131. doi:10.1007/s00894-008-0380-2
Oliveira BG, Vasconcellos MLAA, Olinda RR, Filho EBA (2009) Struct Chem 20:81–90. doi:10.1007/s11224-008-9401-0
Grabowski SJ, Leszczynski J (2009) Chem Phys 355:169–176. doi:10.1016/j.chemphys.2008.12.011
Araújo RCMU, Ramos MN (1998) J Braz Chem Soc 9:499–505
Hobza P, Havlas Z (2000) Chem Rev 100:4253–4264. doi:10.1021/cr990050q
Acknowledgments
The authors gratefully acknowledge partial financial support to CAPES and CNPq Brazilian Funding agencies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oliveira, B.G., Araújo, R.C.M.U., Carvalho, A.B. et al. Small heterocyclics as hydrogen bond acceptors and donors: the case of the C2H3XS···NH3 complexes (X = H, F and CH3). Struct Chem 20, 663–670 (2009). https://doi.org/10.1007/s11224-009-9458-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9458-4