Abstract
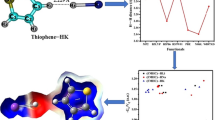
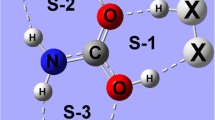
The geometries of three isomers of the C2H4O···2HF tri-molecular heterocyclic hydrogen-bonded complex were examined through B3LYP/aug-cc-pVDZ calculations. Analysis of structural parameters, determination of CHELPG (charge electrostatic potential grid) intermolecular charge transfer, interpretation of infrared stretching modes, and Bader’s atoms in molecules (AIM) theory calculations was carried out in order to characterize the hydrogen bonds in each isomer of the C2H4O···2HF complex. The most stable structure was determined through the identification of hydrogen bonds between C2H4O and HF, (O···H), as well as in the hydrofluoric acid dimer, (HFD–R···HFD). However, the existence of a tertiary interaction (Fλ···Hα) between the fluoride of the second hydrofluoric acid and the axial hydrogen atoms of C2H4O was decisive in the identification of the preferred configuration of the C2H4O···2HF system.

Geometries of three isomers of the C2H4O···2HF tri-molecular heterocyclic hydrogen-bonded complex


Similar content being viewed by others
References
Palmer DC (2004) The chemistry of heterocyclic compounds, vol 60. Wiley, New York
Acheson RM (1976) An introduction to the chemistry of heterocyclic compounds. Wiley, New York
Gritter RJ (1967) The chemistry of the ether linkage. Interscience, New York
Lewars EG (1984) Comprehensive organic chemistry. Pergamon, New York
Legon AC (1995) Chem Phys Lett 247:24–31
Legon AC, Kisiel Z, Georgiou AS, Millen DJ (1989) Chem Phys Lett 155:447–454
Goodwin EJ, Millen DJ, Legon AC (1986) J Chem Phys 85:676–682
Oliveira BG, Araújo RCMU, Carvalho AB, Ramos MN (2007) J Theor Comp Chem 6:647–660
Oliveira BG, Santos ECS, Duarte EM, Araújo RCMU, Ramos MN, Carvalho AB (2004) Spectrochim Acta A 60:1883–1887
Oliveira BG, Duarte EM, Araújo RCMU, Ramos MN, Carvalho AB (2005) Spectrochim Acta A 61:491–494
Everaert GP, Herrebout WA, van der Veken BJ (2000) J Mol Struct 550:399–411
Oliveira BG, Araújo RCMU, Pereira FS, Lima EF, Silva WLV, Carvalho AB, Ramos AB (2008) Quim Nova (in press)
Jursic BS (1998) J Mol Struct (THEOCHEM) 434:37–42
Panek J, Latajka A (2000) Chem Phys Lett 332:617–623
DuPre DB, Yappert MC (2002) J Phys Chem A 106:567–574
Rozas I, Alkorta I, Elguero J (1997) J Phys Chem A 101:9457–9463
Bader RFW (1991) Chem Rev 91:893–928
Oliveira BG, Vanconcellos MLAA (2006) J Mol Struct (THEOCHEM) 774:83–88
Oliveira BG, Araújo RCMU (2007) Quim Nova 30:791–796
Chirlian LE, Francl MM (1987) J Comp Chem 8:894–905
Breneman CM, Wiberg KB (1990) J Comp Chem 11:361–373
Frisch MJ et al (1998) Gaussian 98W (Revision A.1). Gaussian, Pittsburgh, PA
Bader RFW (1990) Atoms in molecules. A quantum theory, Clarendon, Oxford
AIM 2000 1.0 designed by Biegler-König, F; University of Applied Sciences, Bielefeld, Germany
Pauling L (1945) The Nature of the chemical bond. Cornell University Press, New York
Koch U, Popelier PLA (1995) J Phys Chem 99:9747–9754
van Duijneveldt FB, Murrell JN (1967) J Chem Phys 46:1759–1767
Umeyama U, Morokuma K (1977) J Am Chem Soc 99:1316–1332
Oliveira BG, Pereira FS, Araújo RCMU, Ramos MN (2006) Chem Phys Lett 427:181–195
Kollman P, Allen LC (1972) Chem Rev 72:283–303
Hobza P, Havlas Z (2000) Chem Rev 100:4253–4263
Hernandes MZ, da Silva JBP, Longo RL (2002) J Braz Chem Soc 13:36–42
Vasconcellos MLAA, Oliveira BG, Leite LFCC (2008) J Mol Struct (THEOCHEM) (in press)
Oliveira BG, Araújo RCMU, Carvalho AB, Ramos MN, Hernandes MZ, Cavalcante KR (2007) J Mol Struct (THEOCHEM) 802:91–97
Acknowledgments
The authors gratefully acknowledge the partial financial support from the Brazilian Funding agencies CAPES and CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, B.G., Araújo, R.C.M.U., Chagas, F.F. et al. The electronic structure of the C2H4O···2HF tri-molecular heterocyclic hydrogen-bonded complex: a theoretical study. J Mol Model 14, 949–955 (2008). https://doi.org/10.1007/s00894-008-0337-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0337-5