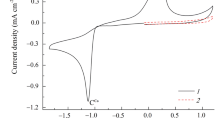
Abstract
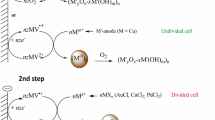
Two-step electrosynthesis of CoO-CoO•xH2O-supported metal nanoparticles (MNPs, M = Au, Ag, Pd) was carried out in N,N-dimethylformamide in the absence and in presence of poly(N-vinylpyrrolidone) (PVP) using atmospheric oxygen as both a reagent and a mediator at potentials of its reduction to a superoxide radical anion. In the first step, oxygen reduction in the presence of Co2+ ions added to the solution as a salt or generated by dissolving the Co-anode during electrolysis produces a mixture of cobalt oxide CoO and its hydrated form CoO-CoO • xH2O (CoOxHy ). When Ag+, Au+, Pd2+ ions are added to the obtained solution of CoOx H y, a redox reaction between CoO and the metal ion gives the MNPs and CoO+. In the second step, oxygen-mediated electroreduction of CoO+ serving as the second mediator is carried out, and the redox reaction is completely shifted towards the formation of MNPs. In the absence of PVP, AgNPs (18±4 nm) bind and stabilize completely in the CoOxHy matrix, PdNPs (6±1 nm) stabilize only partially, and AuNPs (21±10 nm) do not bind and, therefore, only their agglomerates are obtained (~200 nm). In the presence of PVP, individual AgNPs (5±2 nm), AuNPs (13±5 nm), PdNPs (3±1 nm) are stabilized in the PVP shell and are bound by the matrix. The obtained nanocomposites M/CoOx H y and M@PVP/CoOxHy catalyze the reduction of p-nitrophenol with sodium borohydride in an aqueous medium. Their catalytic activity is due to MNPs; CoOx H y acts as an inert matrix.
Similar content being viewed by others
References
A. Eremenko, N. Smirnova, I. Gnatiuk, O. Linnik, N. Vityuk, Y. Mukha, A. Korduban, in Nanocomposites and Polymers with Analytical Methods, Ed. J. Cuppoletti, InTech, Rijeka, 2011, p. 51; DOI: 10.5772/18252.
S. M. Majhi, G. K. Naik, H.-J. Lee, H.-G. Song, C.-R. Lee, I.-H. Lee, Y.-T. Yu, Sensors and Actuators, B, 2018, 268, 223.
J. Liu, S. Zou, S. Li, X. Liao, Y. Hong, L. Xiao, J. Fan, J. Mater. Chem. A, 2013, 1, 4038.
R. P. Padbury, J. C. Halbur, P. J. Krommenhoek, J. B. Tracy, J. S. Jur, Langmuir, 2015, 31, 1135.
D. Han, Z. Zhang, Z. Bao, H. Xing, Q. Ren, Front. Chem. Sci. Eng., 2018, 12, 24.
T. Hu, Y. Wang, Q. Liu, L. Zhang, H. Wang, T. Tang, W. Chen, M. Zhao, J. Jia, Int. J. Hydrogen Energy, 2017, 42, 25951.
F.-Z. Song, Q.-L. Zhu, X. Yang, W.-W. Zhan, P. Pachfule, N. Tsumori, Q. Xu, Adv. Energy Mater., 2018, 8, 1701416.
A. B. Kuriganova, I. N. Leontyev, A. S. Alexandrin, O. A. Maslova, A. I. Rakhmatullin, N. V. Smirnova, Mendeleev Commun., 2017, 27, 67.
A. B. Kuriganova, D. V. Leontyeva, S. Ivanov, A. Bund, N. V. Smirnova, J. Appl. Electrochem., 2016, 46, 1245.
A. B. Kuriganova, N. V. Smirnova, Mendeleev Commun., 2014, 24, 351.
D. E. Doronkin, A. B. Kuriganova, I. N. Leontyev, S. Baier, H. Lichtenberg, N. V. Smirnova, J.-D. Grunwaldt, Catal. Lett., 2016, 146, 452.
P. S. Solmanov, N. M. Maximov, N. N. Tomina, A. A. Pimerzin, Mendeleev Commun., 2018, 28, 562.
A. V. Rassolov, G. N. Baeva, I. S. Mashkovsky, A. Yu. Sta-kheev, Mendeleev Commun., 2018, 28, 538.
S. W. Lee, J. T. Song, J. Kim, J. Oh, J. Y. Park, Nanoscale, 2018, 10, 3911.
Z. Zhang, Q. Wu, X. Bu, Z. Hang, Z. Wang, Q. Wang, Y. Ma, Bull. Korean Chem. Soc., 2018, 39, 71.
M. Liu, W. Tang, Y. Xu, H. Yu, H. Yin, S. Zhao, S. Zhou, Appl. Catal. A, 2018, 549, 273.
P. Supriya, B. T. V. Srinivas, K. Chowdeswari, N. V. S. Naidu, B. Sreedhar, Mater. Chem. Phys., 2018, 204, 27.
M. Gilanizadeh, B. Zeynizadeh, Res. Chem. Intermed., 2018, 44, 6053.
F. Aryanasab, RSC Adv., 2016, 6, 32018.
S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha, T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971.
V. V. Yanilkin, N. V. Nastapova, G. R. Nasretdinova, R. R. Fazleeva, Yu. N. Osin, Electrochem. Commun., 2016, 69, 36.
V. V. Yanilkin, G. R. Nasretdinova, V. A. Kokorekin, Russ. Chem. Rev., 2018, 87, 1080.
V. V. Yanilkin, N. V. Nastapova, R. R. Fazleeva, G. R. Nasretdinova, E. D. Sultanova, A. Yu. Ziganshina, A. T. Gubaidullin, A. I. Samigullina, V. G. Evtyugin, V. V. Vorob’ev, Yu. N. Osin, Russ. J. Electrochem., 2018, 54, 265.
G. R. Nasretdinova, R. R. Fazleeva, Yu. N. Osin, V. G. Evtugin, A. T. Gubaidullin, A. Yu. Ziganshina, V. V. Yanilkin, Electrochim. Acta, 2018, 285, 149.
V. V. Yanilkin, N. V. Nastapova, E. D. Sultanova, G. R. Nasret dinova, R. K. Mukhitova, A. Yu. Ziganshina, I. R. Nizameev, M. K. Kadirov, Russ. Chem. Bull., 2016, 65, 125.
V. V. Yanilkin, N. V. Nastapova, G. R. Nasretdinova, G. M. Fazleeva, L. N. Islamova, Yu. N. Osin, A. T. Gubaidullin, ECS J. Solid State Sci. Technol., 2017, 6, M143.
L. A. Dykman, V. A. Bogatyrev, S. Yu. Shchegolev, N. G. Khlebtsov, Zolotye nanochastitsy. Sintez, svoystva, biomedit-sinskoye primenenie [Gold Nanoparticles. Synthesis, Properties, Biomedical Application], Nauka, Moscow, 2008, 319 pp. (in Russian).
V. V. Yanilkin, R. R. Fazleeva, G. R. Nasretdinova, N. V. Nas tapova, Yu. N. Osin, Butlerov Commun., 2016, 46, 128.
V. V. Yanilkin, R. R. Fazleeva, G. R. Nasretdinova, N. V. Nastapova, Yu. N. Osin, New Mater., Compd. Appl., 2018, 2, 28.
V. V. Yanilkin, R. R. Fazleeva, G. R. Nasretdinova, N. V. Nastapova, Yu. N. Osin, ECS J. Solid State Sci. Technol., 2018, 7, M55.
V. V. Yanilkin, R. R. Fazleeva, N. V. Nastapova, G. R. Nasretdinova, A. T. Gubaidullin, N. B. Berezin, Yu. N. Osin, Russ. J. Electrochem., 2018, 54, 650.
I. P. Suzdalev, Nanotekhnologiya: fiziko-khimiya nanoklasterov, nanostruktur i nanomaterialov [Nanotechnology: Physical Chemistry of Nanoclusters, Nanostructures, and Nanomaterials], 2nd ed., Librokom, Moscow, 2009, 589 pp. (in Russian).
B. I. Kharisov, O. V. Kharissova, U. Ortiz-Méndez, Handbook of Less-Common Nanostructures, CRC Press, Taylor and Francis Group, Boca Raton, 2012, 836 pp.
A. M. Tafesh, J. Weiguny, Chem. Rev., 1996, 96, 2035.
D. Astruc, Nanoparticles and Catalysis, Wiley-VCH, Weinheim, 2008, 663 pp.
P. Lara, K. Philippot, Catal. Sci. Technol., 2014, 4, 2445.
V. V. Yanilkin, N. V. Nastapova, R. R. Fazleeva, G. R. Nasretdinova, E. D. Sultanova, A. Yu. Ziganshina, A. T. Gubaidullin, A. I. Samigullina, V. G. Evtugin, V. V. Vorobev, Yu. N. Osin, Russ. Chem. Bull., 2018, 67, 215.
P. Babji, V. L. Rao, Int. J. Chem. Stud., 2016, 4, 123.
M. Yaseen, Z. Shah, R. C. Veses, S. L. P. Dias, É. C. Lima, G. S. dos Reis, J. C. P. Vaghetti, W. S. D. Alencar, K. Meh-mood, J. Anal. Bioanal. Technol., 2017, 8, 1.
N. Pradhan, A. Pal, T. Pal, Colloids Surf., A, 2002, 196, 247.
P. Hervés, M. Pérez-Lorenzo, L. M. Liz-Marzán, J. Dzubiella, Y. Lu, M. Ballauff, Chem. Soc. Rev., 2012, 41, 5577.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 0241–0254, February, 2020.
Rights and permissions
About this article
Cite this article
Fazleeva, R.R., Nasretdinova, G.R., Osin, Y.N. et al. Two-step electrosynthesis and catalytic activity of CoO−CoO • xH2O-supported Ag, Au, and Pd nanoparticles. Russ Chem Bull 69, 241–254 (2020). https://doi.org/10.1007/s11172-020-2752-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2752-4