Abstract
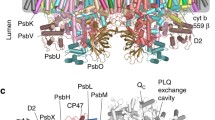
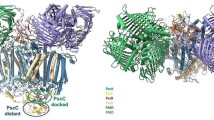
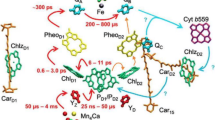
In the photosystem II (PSII) of oxygenic photosynthetic organisms, the reaction center (RC) core mediates the light-induced electron transfer leading to water splitting and production of reduced plastoquinone molecules. The reduction of plastoquinone to plastoquinol lowers PSII affinity for the latter and leads to its release. However, little is known about the role of protein dynamics in this process. Here, molecular dynamics simulations of the complete PSII complex embedded in a lipid bilayer have been used to investigate the plastoquinol release mechanism. A distinct dynamic behavior of PSII in the presence of plastoquinol is observed which, coupled to changes in charge distribution and electrostatic interactions, causes disruption of the interactions seen in the PSII–plastoquinone complex and leads to the “squeezing out” of plastoquinol from the binding pocket. Displacement of plastoquinol closes the second water channel, recently described in a 2.9 Å resolution PSII structure (Guskov et al. in Nat Struct Mol Biol 16:334–342, 2009), allowing to rule out the proposed “alternating” mechanism of plastoquinol–plastoquinone exchange, while giving support to the “single-channel” one. The performed simulations indicated a pivotal role of D1-Ser264 in modulating the dynamics of the plastoquinone binding pocket and plastoquinol–plastoquinone exchange via its interaction with D1-His252 residue. The effects of the disruption of this hydrogen bond network on the PSII redox reactions were experimentally assessed in the D1 site-directed mutant Ser264Lys.







Similar content being viewed by others
References
Antal T, Rubin A (2008) In vivo analysis of chlorophyll a fluorescence induction. Photosynth Res 96:217–226
Antal TK, Osipov V, Matorin DN, Rubin AB (2011) Membrane potential is involved in regulation of photosynthetic reactions in the marine diatom Thalassiosira weissflogii. J Photochem Photobiol B 102:169–173
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98:10037–10041
Barrett CP, Hall BA, Noble ME (2004) Dynamite: a simple way to gain insight into protein motions. Acta Crystallogr D Biol Crystallogr 60:2280–2287
Berendsen HJC, Postma JPM, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Boisvert S, Joly D, Carpentier R (2006) Quantitative analysis of the experimental O-J–I–P chlorophyll fluorescence induction kinetics. FEBS J 273:4770–4777
Bradbrook GM, Gleichmann T, Harrop SJ, Habash J, Raftery J, Kalb J, Yariv J, Hillier IH, Helliwell JR (1998) X-ray and molecular dynamics studies of concanavalin—a glucoside and mannoside complexes-relating structure to thermodynamics of binding. J Chem Soc Faraday Trans 94:1603–1611
Bruccoleri RE, Novotny J, Davis ME, Sharp KA (1997) Finite difference Poisson-Boltzmann electrostatic calculations: Increased accuracy achieved by harmonic dielectric smoothing and charge antialiasing. J Comput Chem. 18(268–276):1997
Cardona T, Sedoud A, Cox N, Rutherford AW (2012) Charge separation in Photosystem II: a comparative and evolutionary overview. Biochim Biophys Acta 1817:26–43
Case DA, Darden TA, Cheatham TA III, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Pearlman DA, Crowley M, Walker RC, Zhang W, Wang B, Hayik S, Roitberg A, Seabra G, Wong KF, Paesani F, Wu X, Brozell S, Tsui V, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Beroza P, Mathews DH, Schafmeister C, Ross WS, Kollman PA (2006) AMBER 9. University of California, San Francisco
Chatterjee R, Milikisiyants S, Coates CS, Lakshmi KV (2011) High resolution two-dimensional 1H and 14N hyperfine sublevel correlation spectroscopy of the primary quinone of Photosystem II. Biochemistry 50:491–501
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N-log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA et al (2004) Gaussian 03, Revision C.02. Gaussian Inc., Wallingford
Giardi MT, Scognamiglio V, Rea G, Rodio G, Antonacci A, Lambreva M, Pezzotti G, Johanningmeier U (2009) Optical biosensors for environmental monitoring based on computational and biotechnological tools for engineering the photosynthetic D1 protein of Chlamydomonas reinhardtii. Biosens Bioelectron 25:294–300
Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W (2009) Cyanobacterial photosystem II at 2.9-A resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol 16:334–342
Hasegawa K, Noguchi T (2014) Molecular interactions of the quinone electron acceptors QA, QB, and QC in photosystem II as studied by the fragment molecular orbital method. Photosynth Res 120:113–123
Hess B, Bekker H, Berendsen HJC, Fraaije JGE (1997) M. LINCS: a linear constraint solver for molecular simulations. J Comp Chem 18:1463–1472
Ho BK, Gruswitz F (2008) HOLLOW: generating accurate representations of channel and interior surfaces in molecular structures. BMC Struct Biol 8:49
Holst M, Saied F (1993) Multigrid solution of the Poisson-Boltzmann equation. J Comput Chem 14:105–113
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65:712–725
Ishikita H, Knapp EW (2005) Control of quinone redox potentials in photosystem II: electron transfer and photoprotection. J Am Chem Soc 127:14714–14720
Joly D, Carpentier R (2009) Sigmoidal reduction kinetics of the photosystem II acceptor side in intact photosynthetic materials during fluorescence induction. Photochem Photobiol Sci 8:167–173
Jorgensen WL, Tirado-Rives J (1988) The OPLS potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J Am Chem Soc 110:1657–1666
Kandt C, Ash WL, Tieleman DP (2007) Setting up and running molecular dynamics simulations of membrane proteins. Methods 41:475–488
Kato Y, Nagao R, Noguchi T (2016) Redox potential of the terminal quinone electron acceptor QB in photosystem II reveals the mechanism of electron transfer regulation. Proc Natl Acad Sci USA 113:620–625
Kern J, Guskov A (2012) Lipids in photosystem II: multifunctional cofactors. J Photochem Photobiol, B 104:19–34
Kühn P, Pieper J, Kaminskaya O, Eckert HJ, Lechner RE, Shuvalov V, Renger G (2005) Reaction pattern of photosystem II: oxidative water cleavage and protein flexibility. Photosynth Res 84:317–323
Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II. Nature 438:1040–1044
Miyamoto S, Kollman PA (1992) SETTLE: an analytical version of the SHAKE and RATTLE algorithms for rigid water models. J Comp Chem 13:952–962
Mobley DL, Chodera JD, Dill KA (2006) On the use of orientational restraints and symmetry number corrections in alchemical free energy calculations. J. Chem. Phys. 125:084902
Müh F, Glöckner C, Hellmich J, Zouni A (2012) Light-induced quinone reduction in photosystem II. Biochim Biophys Acta 1817:44–65
Noguchi T (2015) Spectroscopic analysis of the redox reactions of π -conjugated cofactors in photosynthetic reaction center. In: Akasaka T, Osuka A, Fukuzumi S, Kandori H, Aso Y (eds) Chemical science of π-electron systems. Springer, Tokyo, pp 675–694
Oettmeier W (1999) Herbicide resistance and supersensitivity in photosystem II. Cell Mol Life Sci CMLS 55:1255–1277
Pieper J, Hauss T, Buchsteiner A, Baczyński K, Adamiak K, Lechner RE, Renger G (2007) Temperature- and hydration-dependent protein dynamics in photosystem II of green plants studied by quasielastic neutron scattering. Biochemistry 46:11398–11409
Pospišil P, Dau H (2002) Valinomycin sensitivity proves that light-induced thylakoid voltages result in millisecond phase of chlorophyll fluorescence transients. Biochim Biophys Acta 1554:94–100
Preiss S, Schrader S, Johanningmeier U (2001) Rapid, ATP-degradation of a truncated D1 protein in the chloroplast. Eur J Biochem 268:4562–4569
Reifarth F, Renger G (1998) Indirect evidence for structural changes coupled with QB−. formation in photosystem II. FEBS Lett 428:123–126
Saito K, Rutherford AW, Ishikita H (2013) Mechanism of proton coupled quinone reduction in Photosystem II. Proc Natl Acad Sci USA 110:954–959
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Siu SWI, Vacha R, Jungwirth P, Böckmann RA (2008) Biomolecular simulations of membranes: physical properties from different force fields. J Chem Phys 128:125103
Sproviero EM, Gascón JA, McEvoy JP, Brudvig GW, Batista VS (2007) Quantum mechanics/molecular mechanics structural models of the oxygen-evolving complex of photosystem II. Curr Opin Struct Biol 17:173–180
Srivastava A, Strasser RJ, Govindjee S (1995) Polyphasic rise of chlorophyll a fluorescence in herbicide-resistant D1 mutants of Chlamydomonas reinhardtii. Photosynth Res 43:131–141
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO Approximations and Application to 70 Elements. J Mol Model 13:1173–1213
Stewart JJP (2008) MOPAC2009. Stewart computational chemistry, Colorado Springs, CO, USA
Stirbet A, Govindjee S (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I–P rise. Photosynth Res 113:15–61
Stirbet A, Riznichenko GY, Rubin AB, Govindjee S (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochem (Moscow) 79:291–323
Suga M, Akita F, Hirata F, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen JR (2015) Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517:99–103
Sundby C, Chow WS, Anderson JM (1993) Effects on photosystem II function, photoinhibition, and plant performance of the spontaneous mutation of SerinFe-264 in the photosystem II reaction center D1 protein in Triazine-resistant Brassica napus L. Plant Physiol 103:105–113
Suzuki H, Nagasaka MA, Sugiura M, Noguchi T (2005) Fourier transform infrared spectrum of the secondary quinone electron acceptor Q(B) in photosystem II. Biochemistry 44:11323–11328
Tai K, Shen T, Henchman RH, Bourne Y, Marchot P, McCammon JA (2002) Mechanism of acetylcholinesterase inhibition by fasciculin: a 5-ns molecular dynamics simulation. J Am Chem Soc 124:6153–6161
Trebst A (1991) The molecular basis of resistance of photosystem II inhibitors. In: Casseley JC, Cussans GW, Atkin RK (eds) Herbicide resistance in weeds and crops. Butterworth-Heinemann, Oxford, pp 145–164
Umena Y, Kawakami K, Shen JR, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
van der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible and free. J Comp Chem 26:1701–1718
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25:247–260
Wilski S, Johanningmeier U, Hertel S, Oettmeier W (2006) Herbicide binding in various mutants of the photosystem II D1 protein of Chlamydomonas reinhardtii. Pestic Biochem Physiol 84:157–164
Xin C-P, Yang J, Zhu X-G (2013) A model of chlorophyll a fluorescence induction kinetics with explicit description of structural constraints of individual photosystem II units. Photosynth Res 117:339–354
Acknowledgments
This work was supported by the COST Action TD1102 “PHOTOTECH. Photosynthetic proteins for technological applications: biosensors and biochips” and by the grant ASI Photosynchlamy n 2013-068-R0 to M.T.G.. MD simulations were supported by standard high-performance computing grants from CASPUR. C. reinhardtii PSII D1 S264 K mutant was kindly provided by Prof. Udo Johanningmeier and Dr. Ivo Bertalan from the Institute of Plant Physiology, Martin Luther University, Halle-Wittenberg (Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Veranika Zobnina and Maya D. Lambreva contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zobnina, V., Lambreva, M.D., Rea, G. et al. The plastoquinol–plastoquinone exchange mechanism in photosystem II: insight from molecular dynamics simulations. Photosynth Res 131, 15–30 (2017). https://doi.org/10.1007/s11120-016-0292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0292-4