Abstract
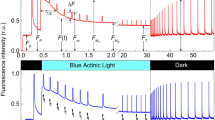
Chlorophyll (Chl) a fluorescence transient, a sensitive and non-invasive probe of the kinetics and heterogeneity of the filling up of the electron acceptor pool of Photosystem II (PS II), was used to characterize D1-mutants of Chlamydomonas reinhardtii. Using a shutter-less system (Plant Efficiency Analyzer, Hansatech, UK), which provides the first measured data point at 10 μs and allows data accumulation over several orders of magnitude of time, we have characterized, for the first time, complete Chl a fluorescence transients of wild type (WT), cell wall less (CW-15) C. reinhardtii and several herbicide-resistant mutants of the D1 proteins: D1-V219IFootnote 1 A251V, F255Y, S264A G256D and L275F. In all cases, the Chl a fluorescence induction transients follow a pattern of O-J-I-P where J and I appear as two steps between the minimum Fo (O) and the maximum Fmax (Fm, P) levels. The differences among the mutants are in the kinetics of the filling up of the electron acceptor pool of PS II (this paper) in addition to those in the re-oxidation kinetics of QA − to QA, published elsewhere (Govindjee et al. (1992) Biochim Biophys. Acta: 1101: 353–358; Strasser et al. (1992) Archs. Sci. Genève 42: 207–224) and not in the ratio of the maximal fluorescence Fm to the initial fluorescence Fo. The value of this experimental ratio is Fm/Fo = 4.4±0.21 independent of the mutation. At 600 W m−2 of 650 nm excitation, distinct hierarchy in the fraction of variable Chl a fluorescence at the J level is observed: S264A > A251V ∼ G256A > L275F ∼ V219I > F255Y ∼ CW-15 ∼ WT. At 300 and 60 W m−2 excitation, a somewhat similar hierarchy among the mutants was observed for the intermediate levels J and I. Addition of bicarbonate-reversible inhibitor formate did not change the O to J phases, slowed the I to P rise, and in many cases, slowed the decay of fluorescence beyond the P level. These observations are interpreted in terms of formate effect being on the acceptor rather than on the donor side (S-states) of PS II. The formate effect was different in different mutants, with L275F being the most insensitive mutant followed by others (V219I, F255Y, WT, A251V and S264A). Further, in the presence of high concentrations of DCMU, identical transients were observed for all the mutants and the WT.
The quantum yield of photochemistry of PS II, calculated from 1-(Fo/Fm), is in the range of 0.73 to 0.82 for the WT as well as for the mutants examined. Thus, in contrast to differences in the kinetics of the electron acceptor side of PS II, there were no significant differences in the maximum quantum yield of PS II, among the mutants tested. We suggest that earlier photochemistry yield values were much lower (0.4−0.6) than those reported here due to either higher measured values of Fo by instruments using camera shutters, or due to the use of cells grown in less than-optimal conditions.
Similar content being viewed by others
Notes
The mutants are labeled as follows: the single letter code for the wild type amino acid, followed by the residue number, then the code for the mutated amino acid.
References
Brewer, PE, Arntzen, CJ and Slife, FW (1979) Effect of atrazine, cyanazine and procyazine on the photochemical reactions of isolated chloroplasts. Weed Science 27: 300–308
Blubaugh, DJ and Govindjee (1988). The molecular mechanism of the bicarbonate effect at the plastoquinone reductase site of photosynthesis. Photosynth Res 19: 85–128
Cao, J, Ohad, N, Hirschberg, J, Xiong, J and Govindjee (1992) Binding affinity of bicarbonate and formate in herbicide-resistant D1 mutants of Synechococcus sp. PCC 7942. Photosynth Res 34: 397–408
Crofts, AR, Baroli, I, Kramer, D and Taoka, S (1993) Kinetics of electron transfer between QA and QB in wild type and herbicideresistant mutants of Chlamydomonas reinhardtii. Z Naturforsch 48c: 259–266
Delosme, R (1967) Étude de l'induction de fluorescence des algues vertes et des chloroplastes au dèbut d'une illumination intense. Biochim Biophys Acta 143: 108–128
Diner, BA, Petrouleas, V and Wendoloski, JJ (1991) The iron quinone electron acceptor complex of Photosystem II. Physiol Plant 81: 423–436
Eaton-Rye, JJ and Govindjee (1988a) Electron transfer through the quinone acceptor complex of Photosystem II in bicarbonatedepleted spinach thylakoid membranes as a function of actinic flash number and frequency. Biochim Biophys Acta 935: 237–247
Eaton-Rye, JJ and Govindjee (1988b) Electron transfer through the quinone acceptor complex of Photosystem II after one or two actinic flashes in bicarbonate-depleted spinach thylakoid membranes. Biochim Biophys Acta 935: 248–257
Eggenberg, P, Schwarz, B and Strasser, RJ (1992) Screening a biotype by fluorescence techniques: Two wavelengths fluorescence amplitude analysis and O-J-I-P fluorescence rise analysis. In: Murata, N (ed) Research in Photosynthesis, Vol 4, pp 611–614. Kluwer Academic Publisher, Dordrecht, the Netherlands
El-Shintinawy, F, Xu, C, and Govindjee (1990) A dual bicarbonate reversible formate effect in Chlamydomonas cells. J Plant Physiol 136: 421–428
Erickson, JM, Pfister, K, Rahire, M, Togasaki, RK, Mets, L and Rochaix JD (1989) Molecular and biophysical analysis of herbicideresistant mutants of Chlamydomonas reinhardtii: Structure-function relationship of the Photosystem II D1 polypeptide. The Plant Cell 1: 361–371
Gorman, DS and Levine, RP (1965) Cytochrome a and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 54: 1665–1669
Govindjee and Papageorgiou, G (1971) Chlorophyll fluorescence and photosynthesis: Fluorescence transients. In: Giese, AC (ed) Photophysiology, Vol 6, pp 1–50. Academic Press, New York
Govindjee and Van, Rensen, JJS (1993) Photosystem II reaction center and bicarbonate. In: Deisenhofer, J and Norris, J (eds) The photosynthetic Reaction Center, Vol 1, pp 357–389. Academic Press, San Diego, CA
Govindjee and Satoh, K (1986) Fluorescence properties of chlorophyll b and chlorophyll c containing algae. In: Govindjee, Amesz J and Fork, DC (eds) Light Emission by Plants and Bacteria, pp 497–537, Academic Press, New York
Govindjee, Pulles, MPJ, Govindjee, R, van, Gorkom, HJ and Duysens LNM (1976) Inhibition of reoxidation of the secondary acceptor of Photosystem II by bicarbonate depletion. Biochim Biophys Acta 449: 602–605
Govindjee, Vernotte, C, Peteri, B, Astier, C and Etienne, A-L (1990) Differential sensitivity of bicarbonate-reversible formate effects on herbicide-resistant mutants of Synechocystis 6714. FEBS Lett 267: 273–276
Govindjee, Schwarz, B, Rochaix, JD and Strasser, RJ (1991) The herbicide-resistant D1 mutant L275F of Chlamydomonas reinhardtii fails to show the bicarbonate-reversible formate effect on chlorophyll a fluorescence transients. Photosynth Res 27: 199–208
Govindjee, Eggenberg, P, Pfister, K and Strasser, RJ (1992) Chlorophyll a fluorescence decay in herbicide-resistant D1 mutants of Chlamydomonas reinhardtii and the formate effect. Biochim Biophys Acta 1101: 353–358
Guenther, JE, Nemson, JA and Melis, A (1990) Development of Photosystem II in dark-grown Chlamydomonas reinhardtii. A light-dependent conversion of PS IIβ, QB-non-reducing centers to the PS IIα, QB reducing form. Photosynth Res 24: 35–46
Hsu, BD (1993) Evidence for the contribution of the S-state transitions of oxygen evolution to the initial phase of fluorescence induction. Phtosynth Res 36: 81–88
Krause, GH and Weis, E (1991) Chlorophyll fluorescence and photosynthesis: The basics. Ann Rev Plant Physiol Plant Mol Biol 42: 313–349
Lavergne, J and Joliot, P (1991) Restricted diffusion in photosynthetic membranes. Trends Bio Sci 16: 129–134
Lavorel, J and Etienne, A-L (1977) In vivo chlorophyll fluorescence. Topics in Photosynthesis 2: 203–268
Neubauer, C and Schreiber, U (1987) The polyphasic rise of chlorophyll fluorescence upon the onset of strong continuous illumination: I. Staturation characteristics and partial control by the Photosystem II acceptor side. Z Naturforsch 42c: 1246–1254
Oettmeier, W (1992) Herbicides of Photosystem II. Topics in Photosynthesis 11: 349–408
Papageorgiou, G (1975) Chlorophyll fluorescence: An intrinsic probe of photosynthesis. In: Govindjee (ed) Bioenergetics of Photosynthesis, pp 320–366. Academic Press, New York
Schreiber, U and Neubauer, C (1987) The polyphasic rise of chlorophyll fluorescence upon the onset of strong continuous illumination: II. Partial control by the Photosystem II donor side and possible ways of interpretation. Z Naturforsch 42c: 1255–1264
Shinkarev, VP and Govindjee (1993) Insight into the relationship of chlorophyll a fluorescence yield to the concentration of its natural quenchers in oxygenic photosynthesis. Proc Natl Acad Sci USA 90: 7466–7469
Spalding, MH, Critchley, C, Govindjee and Ogren, WL (1984) Influence of carbondioxide concentration growth on fluorescence induction characteristic of the green algae Chlamydomonas reinhardtii. Photosynth Res 5: 169–176
Strasser, RJ and Govindjee (1991) The Fo and the O-J-I-P fluorescence rise in higher plants and algae. In: Argyroudi-Akoyunoglou, JH (ed) Regulation of Chloroplast Biogenesis, pp 423–426. Plenum Press, New York
Strasser, R.J and Govindjee (1992) On the O-J-I-P fluorescence transients in leaves and D1 mutants of Chlamydomonas reinhardtii, In: Murata, N (ed) Research in Photosynthesis, Vol II, pp 29–32. Kluwer Academic Publishers, Dordrecht, the Netherlands
Strasser, RJ, Eggenberg, P, Pfister, K and Govindjee (1992) An equilibrium model for electron transfer in Photosystem II acceptor complex: An application to Chlamydomonas reinhardtii cells of D1 mutants and those treated with formate. Archs Sci Genève 42: 207–224
Strasser, RJ, Srivastava, A and Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61: 32–42
Trebst, A (1991) The molecular basis of resistance of Photosystem II herbicides. In: Caseley, JC, Cussans, GW and Atkins, RK (eds) Herbicide Resistance in Weeds and crops, pp 145–164. Butterworth-Heinemann Ltd., Oxford
Velthuys, B (1981) Electron dependent competition between plastoquinone and inhibirors for binding to Photosystem II. FEBS Lett 126: 277–281
Xu, C, Taoka, S, Crofts, AR and Govindjee (1991) Kinetic characteristic of formate/formic acid binding at the plastoquinone reductase site in spinach chloroplasts. Biochim Biophys Acta 1098: 32–40
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Srivastava, A., Strasser, R.J. & Govindjee Polyphasic rise of chlorophyll a fluorescence in herbicide-resistant D1 mutants of Chlamydomonas reinardtii . Photosynth Res 43, 131–141 (1995). https://doi.org/10.1007/BF00042970
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042970