Abstract
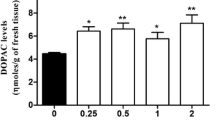
Our aim was to study the specific role of the postsynaptic D1 receptors on dopaminergic response and analyze the metabolized dopamine (DA) in the rat striatum. We used male Wistar rats to evaluate the effects of different doses of a D1 agonist (SKF-38393) and a D1 antagonist (SCH-23390), and their co-administration. The levels of DA and L-3, 4-dihydroxyphenylacetic acid (DOPAC) were measured using high performance liquid chromatography. The systemic injection of SKF-38393 alone at 1, 5 and 10 mg/kg did not alter the DA and DOPAC levels or the DOPAC/DA ratio. In contrast, injection of SCH-23390 alone at 0.25, 0.5 and 1 mg/kg significantly increased the DA and DOPAC levels, as well as the DOPAC/DA ratio, compared with the respective control groups. The co-administration of SCH-23390+SKF-38393 did not alter the DA or DOPAC levels, but it did significantly inhibit the SCH-23390-induced increase of the DA and DOPAC levels. The SCH-23390+SKF-38393 and the SCH-23390-only groups showed an increase in the DOPAC/DA ratio. The co-administration of SCH-23390+PARGYLINE significantly decreased the DOPAC levels and the DOPAC/DA ratio compared with the control and SCH-23390 groups. Taken together, our results showed that selective inhibition with SCH-23390 produced an increase in metabolized DA via striatal monoamine oxidase. These findings also contribute to the understanding of the role of postsynaptic D1 receptors in the long-loop negative feedback system in the rat striatum.
Similar content being viewed by others
References
Groenewegen HJ (2003) The basal ganglia and motor control. Neural Plast 10:107–120
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60:543–554
Bolam JP, Hanley JJ, Booth PAC, Bevan MD (2000) Synaptic organisation of the basal ganglia. J Anat 196:527–542
Jackson DM, Westlind-Danielsson A (1994) Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther 64:291–370
Palermo-Neto J (1997) Dopaminergic systems. Dopamine receptors. Psychiatr Clin N Am 20:705–721
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30:228–235
Brazhnik E, Shah F, Tepper JM (2008) GABAergic afferents activate bothGABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo. J Neurosci 28:10386–10398
Imperato A, Di Chiara G (1988) Effects of locally applied D-1 and D-2 receptor agonists and antagonists studied with brain dialysis. Eur J Pharmacol 156:385–393
Rahman S, McBride JW (2001) D1-D2 dopamine receptor interaction within the nucleus accumbens mediates long-loop negative feedback to the ventral tegmental area (VTA). J Neurochem 77:1248–1255
Saklayen SS, Mabrouk OS, Pehek EA (2004) Negative feedback regulation of nigrostriatal dopamine release: mediation by striatal D1 receptors. J Pharmacol Exp Ther 311:342–348
Bueno-Nava A, González-Piña R, Ávila-Luna A, Alfaro-Rodríguez A (2011) Paradigm of negative feedback via long-loop in the striatal dopamine release modulation in the rat. Rev Neurol 52:371–377
Tomiyama K, Koshikawa N, Funada K, Oka K, Kobayashi M (1995) In vivo microdialysis evidence for transient dopamine release by benzazepines in rat striatum. J Neurochem 65:2790–2795
Saigusa T, Aono Y, Sekino R, Uchida T, Takada K, Oi Y, Koshikawa N (2009) Contribution of vesicular and cytosolic dopamine to the increased striatal dopamine efflux elicited by intrastriatal injection of SKF38393. Eur J Pharmacol 624:10–15
Sekino R, Saigusa T, Aono Y, Uchida T, Takada K, Oi Y, Koshikawa N (2010) Dopamine D1-like receptors play only a minor role in the increase of striatal dopamine induced by striatally applied SKF38393. Eur J Pharmacol 648:80–86
Zackheim JA, Abercrombie ED (2001) Decreased striatal dopamine efflux after intrastriatal application of benzazepine-class D1 agonists is not mediated via dopamine receptors. Brain Res Bull 54:603–607
Abraini JH, Fechtali T, Rostain JC (1994) Lasting effects of dopamine receptor agonists upon striatal dopamine release in free-moving rats: an in vivo voltammetric study. Brain Res 642:199–205
Imperato A, Mulas A, Di Chiara G (1987) The D-1 antagonist SCH23390 stumulates while the D-1 agonist SKF38393 fails to affect dopamine release in the dorsal caudate of freely moving rats. Eur J Pharmacol 142:177–181
LGJr Harsing (2008) Dopamine and the dopaminergic systems of the brain. In: Lajtha A (ed) The handbook of neurochemistry and molecular neurobiology: neural membranes and transport, 3rd edn. Springer, Berlin, pp 149–170
Besson MJ, Cheramy A, Fetz P, Glowinski J (1969) Release of newly synthesized dopamine from dopamine-containing terminals in the striatum of the rat. Proc Natl Acad Sci USA 62:741–748
Besson MJ, Cheramy A, Gauchy C, Glowinski J (1973) In vivo continuous estimation of 3H-DA synthesis an release in the cat caudate nucleus:effects of α-MPT and transaction of the nigro neostriatal pathway. Naunyn Schmiedebergs Arch Pharmacol 278:101–105
Glowinski J (1975) Properties and functions of intraneuronal monoamine compartments in central aminergic neurons. In: Iversen LL, Iversen SD, Snyder SH (eds) The handbook of psychopharmacology 3. Plenum Press, New York, pp 139–167
Cooper JR, Bloom FE, Roth RH (1996) The biochemical basis of neuropharmacology, 7th edn. Oxford University Press, New York/Oxford, pp 293–351
Olfert ED, Cross BM, Mc William AA (1993) Guide for the care and use of experimental animals. Can Counc Anim Care 1:211
Festing MF (1994) Reduction of animal use: experimental design and quality of experiments. Lab Anim 28:212–221
Bueno-Nava A, Gonzalez-Pina R, Alfaro-Rodriguez A (2010) Iron-dextran injection into the substantia nigra in rats decreases striatal dopamine content ipsilateral to the injury site and impairs motor function. Metab Brain Dis 25:235–239
Zetterström T, Sharp T, Collin AK, Ungerstedt U (1988) In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol 148:327–334
Pan JT, Lookingland KJ, Moore KE (1995) Differential effects of corticotropin-releasing hormone on central dopaminergic and noradrenergic neurons. J Biomed Sci 2:50–56
Rasheed N, Ahmad A, Pandey CP, Chaturvedi RK, Lohani M, Palit G (2010) Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem Res 35:22–32
Kalivas PW, Duffy P, Latimer LG (1987) Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther 242:757–763
Acknowledgments
This study is from the first author as a member of the Doctorate Program in Biological Science at the Universidad Autónoma Metropolitana Iztapalapa-Xochimilco. Bueno-Nava was supported by the scholarship No. 47133 from CONACYT in Mexico. The technical assistance of J.L. Cortés-Altamirano and I. Villafaña-Rivera is greatly appreciated. The authors wish to thank Dra. Ivonne M. Heuze de Icasa for his support with the experimental animals.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bueno-Nava, A., Gonzalez-Pina, R., Alfaro-Rodriguez, A. et al. The Selective Inhibition of the D1 Dopamine Receptor Results in an Increase of Metabolized Dopamine in the Rat Striatum. Neurochem Res 37, 1783–1789 (2012). https://doi.org/10.1007/s11064-012-0790-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0790-5