Abstract
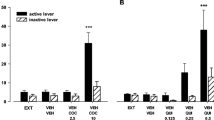
The striatum is known to possess high levels of D1-like and D2-like receptors (D1Rs and D2Rs, respectively). We have previously shown that selective inhibition of D1Rs increases the dopaminergic metabolic response and proposed that this effect is associated with the concomitant activation of postsynaptic D2Rs by endogenous dopamine (DA). Here, we examined whether activation of D2Rs modulates the metabolism and synthesis of DA in the striatum. We used male Wistar rats to evaluate the effects of the systemic administration of a D2R agonist (bromocriptine), a D1R antagonist (SCH-23390), and the co-administration of these compounds with pargyline on the inhibition of monoamine oxidase. DA and l-3,4-dihidroxyphenylacetic acid (DOPAC) levels and 3,4-dihydroxy-l-phenylalanine (l-DOPA) content were measured using high performance liquid chromatography. The systemic administration of SCH-23390 alone, at 0.25, 0.5, 1 or 2 mg/kg, significantly (P < 0.05) increased DOPAC levels and the DOPAC/DA ratio. At 2, 4 and 8 mg/kg, the administration of bromocriptine alone significantly (P < 0.05) decreased DOPAC levels, l-DOPA content and the DOPAC/DA ratio, whereas at 2 mg/kg, it decreased DA levels. In both groups, co-administration of either SCH-23390 or bromocriptine with pargyline decreased DOPAC levels and the DOPAC/DA ratio by approximately 70 % compared to the levels observed in the control groups. In conclusion, administration of the D2R agonist bromocriptine decreased dopaminergic synthesis and metabolism in the striatum; in contrast, administration of the D1R antagonist SCH-23390 induced the opposite effects.



Similar content being viewed by others
References
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60(4):543–554
O’Neill C, Evers-Donnelly A, Nicholson D, O’Boyle KM, O’Connor JJ (2009) D-2 receptor-mediated inhibition of dopamine release in the rat striatum in vitro is modulated by CB1 receptors: studies using fast cyclic voltammetry. J Neurochem 108(3):545–551
Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O (1988) Cloning and expression of a rat D2 dopamine receptor cDNA. Nature 336(6201):783–787
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78(1):189–225
Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB (1991) Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350(6319):614–619
Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350(6319):610–614
Cubeddu LX, Hoffmann IS (1982) Operational characteristics of the inhibitory feedback mechanism for regulation of dopamine release via presynaptic receptors. J Pharmacol Exp Ther 223(2):497–501
Schmitz Y, Schmauss C, Sulzer D (2002) Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci 22(18):8002–8009
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30(5):228–235
Bolam JP, Hanley JJ, Booth PA, Bevan MD (2000) Synaptic organisation of the basal ganglia. J Anat 196(Pt 4):527–542
Koos T, Tepper JM (1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2(5):467–472
Mallet N, Le Moine C, Charpier S, Gonon F (2005) Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25(15):3857–3869
Imperato A, Di Chiara G (1988) Effects of locally applied D-1 and D-2 receptor agonists and antagonists studied with brain dialysis. Eur J Pharmacol 156(3):385–393
Rahman S, McBride WJ (2001) D1–D2 dopamine receptor interaction within the nucleus accumbens mediates long-loop negative feedback to the ventral tegmental area (VTA). J Neurochem 77(5):1248–1255
Saklayen SS, Mabrouk OS, Pehek EA (2004) Negative feedback regulation of nigrostriatal dopamine release: mediation by striatal D1 receptors. J Pharmacol Exp Ther 311(1):342–348
Bueno-Nava A, Gonzalez-Pina R, Avila-Luna A, Alfaro-Rodriguez A (2011) Paradigm of negative feedback via long-loop in the striatal dopamine release modulation in the rat. Rev Neurol 52(6):371–377
Bueno-Nava A, Gonzalez-Pina R, Alfaro-Rodriguez A, Avila-Luna A, Arch-Tirado E, Alonso-Spilsbury M (2012) The selective inhibition of the D-1 dopamine receptor results in an increase of metabolized dopamine in the rat striatum. Neurochem Res 37(8):1783–1789
Jaber M, Robinson SW, Missale C, Caron MG (1996) Dopamine receptors and brain function. Neuropharmacology 35(11):1503–1519
Beaulieu J-M, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63(1):182–217
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24(1):125–132
Takashima H, Tsujihata M, Kishikawa M, Freed WJ (1999) Bromocriptine protects dopaminergic neurons from levodopa-induced toxicity by stimulating D(2)receptors. Exp Neurol 159(1):98–104
Bonuccelli U, Del Dotto P, Rascol O (2009) Role of dopamine receptor agonists in the treatment of early Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 4):S44–S53
Olfert E, Cross B, Mc William A (1993) Guide for the care and use of experimental animals. Can Counc Anim Care 1:211
Festing MF (1994) Reduction of animal use: experimental design and quality of experiments. Lab Anim 28(3):212–221
Harsing LGJ (2008) Dopamine and the dopaminergic systems of the brain: neurotransmitter systems. In: Lajtha A, Vizi ES (ed) Handbook of neurochemistry and molecular neurobiology, 3rd edn. Springer, New York, pp 149–170
Alfaro-Rodriguez A, Alonso-Spilsbury M, Arch-Tirado E, Gonzalez-Pina R, Arias-Montano J-A, Bueno-Nava A (2013) Histamine H-3 receptor activation prevents dopamine D-1 receptor-mediated inhibition of dopamine release in the rat striatum: a microdialysis study. Neurosci Lett 552:5–9
Henry B, Crossman AR, Brotchie JM (1998) Characterization of enhanced behavioral responses to l-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp Neurol 151(2):334–342
Prikhojan A, Brannan T, Yahr MD (2000) Comparative effects of repeated administration of dopamine agonists on circling behavior in rats. J Neural Transm (Vienna, Austria : 1996) 107(10):1159–1164
Lindgren HS, Rylander D, Ohlin KE, Lundblad M, Cenci MA (2007) The “motor complication syndrome” in rats with 6-OHDA lesions treated chronically with l-DOPA: relation to dose and route of administration. Behav Brain Res 177(1):150–159
Lane EL, Dunnett SB (2010) Pre-treatment with dopamine agonists influence l-DOPA mediated rotations without affecting abnormal involuntary movements in the 6-OHDA lesioned rat. Behav Brain Res 213(1):66–72
Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y (1995) Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. Eur J Pharmacol 284(1–2):83–91
Imperato A, Mulas A, Di Chiara G (1987) The D-1 antagonist SCH 23390 stimulates while the D-1 agonist SKF 38393 fails to affect dopamine release in the dorsal caudate of freely moving rats. Eur J Pharmacol 142(1):177–181
Mura A, Jackson D, Manley MS, Young SJ, Groves PM (1995) Aromatic l-amino acid decarboxylase immunoreactive cells in the rat striatum: a possible site for the conversion of exogenous l-DOPA to dopamine. Brain Res 704(1):51–60
Lloyd K, Hornykiewicz O (1970) Parkinson’s disease: activity of l-DOPA decarboxylase in discrete brain regions. Science (New York, NY) 170(3963):1212–1213
Ogawa N, Tanaka K, Asanuma M (2000) Bromocriptine markedly suppresses levodopa-induced abnormal increase of dopamine turnover in the parkinsonian striatum. Neurochem Res 25(6):755–758
Fekete MI, Herman JP, Kanyicska B, Palkovits M (1979) Dopamine, noradrenaline and 3,4-dihydroxyphenylacetic acid (DOPAC) levels of individual brain nuclei, effects of haloperidol and pargyline. J Neural Transm 45(3):207–218
Berry MD (2011) The effects of pargyline and 2-phenylethylamine on D1-like dopamine receptor binding. J Neural Transm 118(7):1115–1118
Acknowledgments
The authors wish to thank Dr. Ivonne M. Heuze de Icasa and Dr. Emilio E. Quintana for support with the experimental animals. We thank MVZ. Hugo Lecona Butrón for support with the housing, care, maintenance and monitoring of the health of the experimental animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avila-Luna, A., Prieto-Leyva, J., Gálvez-Rosas, A. et al. D1 Antagonists and D2 Agonists Have Opposite Effects on the Metabolism of Dopamine in the Rat Striatum. Neurochem Res 40, 1431–1437 (2015). https://doi.org/10.1007/s11064-015-1611-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1611-4