Abstract
Purpose
Intraoperative radiation therapy (IORT) is an emerging alternative to adjuvant stereotactic external beam radiation therapy (EBRT) following resection of brain metastases (BM). Advantages of IORT include an instant prevention of tumor regrowth, optimized dose-sparing of adjacent healthy brain tissue and immediate completion of BM treatment, allowing an earlier admission to subsequent systemic treatments. However, prospective outcome data are limited. We sought to assess long-term outcome of IORT in comparison to EBRT.
Methods
A total of 35 consecutive patients, prospectively recruited within a study registry, who received IORT following BM resection at a single neuro-oncological center were evaluated for radiation necrosis (RN) incidence rates, local control rates (LCR), distant brain progression (DBP) and overall survival (OS) as long-term outcome parameters. The 1 year-estimated OS and survival rates were compared in a balanced comparative matched-pair analysis to those of our institutional database, encompassing 388 consecutive patients who underwent adjuvant EBRT after BM resection.
Results
The median IORT dose was 30 Gy prescribed to the applicator surface. A 2.9% RN rate was observed. The estimated 1 year-LCR was 97.1% and the 1 year-DBP-free survival 73.5%. Median time to DBP was 6.4 (range 1.7–24) months in the subgroup of patients experiencing intracerebral progression. The median OS was 17.5 (0.5-not reached) months with a 1 year-survival rate of 61.3%, which did not not significantly differ from the comparative cohort (p = 0.55 and p = 0.82, respectively).
Conclusion
IORT is a safe and effective fast-track approach following BM resection, with comparable long-term outcomes as adjuvant EBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the course of their disease, up to 40% of cancer patients develop brain metastases (BM) [1]. With novel therapeutic options prolonging their overall survival (OS) [2,3,4,5], the diagnostic incidence of BM and risk of local recurrence are increasing [6, 7]. Although BMs do nowadays not necessarily impact overall survival [8, 9], local treatment is critical to prevent or stabilize neurological deterioration and impairment of quality of life (QOL) [10, 11]. If feasible, large or symptomatic lesions require surgical intervention. Adjuvant radiation therapy (RT) of both potential tumor remnants and the resection cavity improves local control rates (LCR) [12,13,14]. Considering the tumor localization, histology and volumes, common RT regimens apply stereotactic external-beam RT (EBRT) of one (stereotactic radiosurgery, SRS) to seven fractions (fractionated stereotactic radiotherapy, FSRT) either before resection or afterwards, following adequate wound healing and recovery from surgery [14,15,16,17,18]. As an alternative, low-energy intraoperative RT (IORT) has increasingly gained attention in the past years. Initial reports suggest promising LCR [19, 20] and a favorable safety profile [21] with a comparatively lower radiation necrosis (RN) incidence [22]. Available data are solely based on retrospective single institution experiences, with a radiation oncology focus on dosage and technical aspects of the IORT approach [23, 24]. Nonetheless, its safety profile has been previously explored in brain tissue for treating glioblastoma [25, 26] and its efficacy is currently evaluated in a phase III trial (NCT02685605). Several advantages of IORT include a steep dose gradient, improved healthy brain tissue sparing [27] and avoiding RT target-volume delineation challenges caused by post-surgery tissue alterations. The instant application of local high dose RT to the tumor bed may prevent early repopulation of residual microscopic tumor. Furthermore, an accelerated completion of the interdisciplinary BM treatment eases a faster recovery, shorter in hospital-times and earlier initiation of subsequent systemic treatments.
We previously reported on a favorable perioperative safety profile of patients receiving IORT for BM in a matched-pair fashion with 388 BM patients who underwent conventional post-surgical RT [28]. Here, we report on their clinical long-term outcome and assess their survival in comparison to the same matched institutional cohort.
Methods
Patients
The study collected data from consecutively recruited patients admitted to the Neurosurgical Department of the University Hospital Bonn between November 2020 and October 2021, who had undergone surgical resection of BM combined with IORT. In all cases, BM were histopathologically confirmed. At a weekly tumor board meeting, interdisciplinary consensus was used to determine the treatment strategies for each patient individually [29]. Treatment plans were also coordinated with the referring physicians and considered the patient’s past oncological therapies. Besides receiving a histopathological diagnosis in case of cancer of unknown primary, criteria for surgical resection were presence or severe risk of acute neurological impairment or clinically significant mass effects as abnormal intracranial pressure or hemispheric shift. In case of multiple BMs, only the clinically manifest lesion was considered for surgical removal to prevent mass effects and tumor-related hydrocephalus [28, 30]. Clinical inclusion criteria for IORT were gross total resection, intraoperative confirmation of BM on frozen tumor sections, no previous intracerebral irradiation and fulfillment of dose constraints as described below. The data were prospectively collected and managed using SPSS (version 25, IBM Corp., Armonk, NY). Informed consent was obtained from all patients. The collected information included, among others, sociodemographic characteristics, primary tumor location, radiological and histopathological characteristics of the intracranial metastatic lesions, baseline functional status. The Karnofsky performance score (KPS) was used to classify the patients according to their functional status at admission. A stratification cut-off of 70 was chosen according to Péus et al. with regard to the patient’s ability to carry on their normal activity and work [31]. Diagnostic-Specific Graded Prognostic Assessment (DS-GPA) [32] scores were calculated by standard procedures. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital Bonn (approval number: 018/21 and 057/22).
IORT
Preoperative contrast-enhanced T1-weighted MRI imaging was used to provide 3D image guidance for both surgery and radiation treatments. Optic nerves, chiasm, and brain stem were identified preoperatively and intraoperatively as organs at risk (OARs) for IORT and delivered doses were defined based on dose-depth template profiles corresponding to each applicator diameter. The INTRABEAM® 600 (Carl Zeiss Meditec AG, Oberkochen, Germany) was used to deliver IORT with a spherical applicator ranging from 1.5 to 5.0 cm diameter by application of nominal 50 kV photons at a standard dose of 30 Gy prescribed to the applicator surface. Decreasing the prescribed dose down to 16 Gy was acceptable in case of OAR doses exceeding the constraints of 12 Gy to the optical system or 12.5 Gy to the brain stem following the QUANTEC (Quantitative Analyses of Normal Tissue Effects in the Clinic) recommendations [33] considering the specific (1.3–1.5 times higher) RBE of low energy photons. In individual cases, an anatomical positioning of the applicator required consideration of further OAR that were not regularly assessed, e.g., cochlea or thalamus, with equal consideration of the QUANTEC recommendations.
Follow-up
All patients had regular follow-up (FU) visits including physical examination and magnetic resonance imaging (MRI). MRI assessments were performed according to the RANO criteria by board-certified radiologists. In case of uncertain clinical or radiographic response, the interdisciplinary neuro-oncological tumor board was consulted and a combined decision was taken upon findings. The following conditions qualified for diagnosis of RN: (1) after initial suspected progressive disease (PD), a minimum of two FU MRI time points showed no sign of ongoing PD; (2) advanced MRI incorporating dynamic susceptibility contrast (DSC) or diffusion weighted imaging (DWI) was concordantly suggestive of RN; (3) RN was confirmed histopathologically after surgery.
Study endpoints
The primary endpoints of the study were RN rates and cumulative 1 year-LCR. The secondary endpoints were DBP, 1 year-OS rates and estimated OS. Local control was defined as the absence of MRI-radiographic PD in or surrounding the previously irradiated BM resection cavity and calculated from the day of surgery until the date of PD. Patients lost to FU or deceased prior to radiographic progression were censored at the last FU time point. OS was defined as the time interval between the date of surgery and the date of either the last FU (censored) or death.
Matching procedure
The study performed a propensity score matching, which involved matching a cohort of 35 patients who received IORT with a cohort of 388 patients who underwent surgery for BM followed by EBRT (patient characteristics provided in Suppl. Table 1). The matching was performed at a ratio of 1:2, and the statistical computing program R (version 4.1.2; The R Foundation for Statistical Computing, https://www.r-project.org/) was used for the analysis as previously described [28]. The group of EBRT patients included all patients aged 18 years or older who underwent surgery for BM at the University Hospital Bonn neuro-oncological center between 2013 and 2018, and who did not receive IORT but EBRT (SRS, FSRT or whole brain radiotherapy (WBRT)) during that period. The study aimed to increase the robustness of the data by selecting known prognostic parameters, such as age [34], KPS and Charlson comorbidity index (CCI) at admission [34,35,36], tumor entity, and the status of solitary versus multiple BM [35], for matching. The balance of these parameters was measured and visualized to ensure that the two groups were comparable. A jitter plot was used to display the distribution of propensity scores. The study protocol for retrospective data collection was approved by the local Ethics committee (approval number: 250/19 and 057/22).
Statistics
The computer software packages used for the data analyses were SPSS and GraphPad Prism (version 9, GraphPad Software, Boston, MA). Fisher’s exact test was used to analyze categorical variables, which were presented in contingency tables. The Mann–Whitney U test was used to compare continuous variables, as the data were not normally distributed. Statistical significance was defined as a p-value of less than 0.05.
Results
Patient and tumor characteristics
Between November 2020 and October 2021, 35 consecutive BM patients receiving IORT to the resection cavity were enrolled. Their median age was 63 (range 43–80) years and the median KPS was 80 (50–100). Of note, 29% of patients had a KPS < 70. The median DS-GPA score was 2 (0–4). The most frequent BM localization was the frontal lobe (37.1%) followed by the occipital lobe (25.7%). Most histopathology results corresponded to non-small cell lung cancer (NSCLC, 54%), followed by melanoma (11%) and breast cancer (6%). With a range of 2 to 10 intracranial lesions, 15 patients (43%) suffered from multiple BM at the time of surgery. Further details on patient characteristics can be found in Table 1.
Treatment and dosimetry
No dose constraints were exceeded and all patients completed treatment. The median IORT duration was 18:12 (6:56–49:00) min and the median prescription dose was 30 (16–30) Gy. The median applicator size was 2.5 (1.5–5.0) cm. The brainstem and the optic tracts (optic nerves and chiasm) were regularly assessed as OARs. Doses to other structures were negligible and therefore not considered relevant for this report. The median distance from the applicator surface was 35.5 (10–65) mm to the brainstem and 60 (13–70) mm to the optic tracts, with a median estimated OAR dose exposure of 0.7 (0.0–6.0) Gy and 0.0 (0.0–4.4) Gy, respectively.
Radiation necrosis rate, local tumor control and distant brain progression
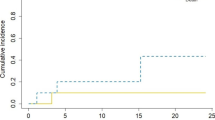
In all patients, a gross total resection was achieved. After a median FU of 10.4 (0.5–24.5) months and a median imaging FU of 7.9 (0.1–24.4) months incorporating a median of 6 (1–13) MRI assessments, only one RN event was noted at 18.7 months. Hence, an overall RN rate of 2.9% was observed (Fig. 1a). As this patient’s RN was a grade 2 event, only mild conservative management was initiated and subsequently led to clinical remission. Of note, the patient did not experience distant intracranial progression and is still alive and systemically stable after 23.2 months of FU.
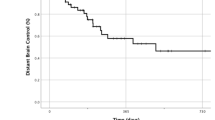
A second patient showed local recurrence after 2.9 months, in addition to previous distant intracranial progression. The latter led to clinical deterioration and subsequent exitus. The overall IORT 1-year LCR was 97.1% (Fig. 1b). With an overall distant brain progression rate of 29.4%, the median DBP-free survival (DBPS) was 24 (0.5-not reached) months and the 1 year-DBPS 73.5% (Fig. 1c). The median time to DBP was 6.4 (range 1.7–24) months in the subgroup of patients experiencing distant intracranial progression. Leptomeningeal spread occurred in 5.7% of cases (2 cases), after 18.2 and 21.9 months, respectively.
Survival and comparison to matched EBRT cohort
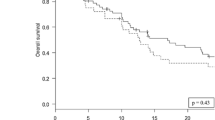
For the IORT cohort, the median OS was 17.5 (0.5-not reached) months and the 1 year-survival rate 61.3% (Fig. 1d). 70 patients from the institutional database of patients, who underwent surgery with subsequent EBRT (Suppl. Table 1) and individually corresponded to the present series were matched at a ratio of 1:2 to those receiving IORT (Fig. 2). The two patient populations did not differ significantly by the matching variables age (p = 0.74), KPS (p = 0.88), primary site of cancer (p = 1.00) and frequency of multiple BM (p = 0.68). Concomitant systemic treatment was equally distributed (p = 0.99). With 61.3% versus 68.2%, the 1 year-survival was not significantly different between IORT and EBRT, respectively (p = 0.82; Table 2). Furthermore, the median OS was comparable with 17.5 months and 26 months, in each respective cohort (p = 0.55; Fig. 3).
Graphical visualization of the applied matching procedure. a Comparative matched pair analysis at a ratio of 1:2 identifies 70 out of 388 patients with resected BM not receiving IORT who individually correspond to the present series of 35 patients with resected BM undergoing IORT. Heat map as color-coded illustration of the matching strategy of patients not receiving IORT to IORT cases stratified by age, KPS at admission, tumor entity and solitary versus multiple BM as matching parameters. The red box illustrates individually-matched patients without IORT. b Love plot demonstrating the balance of the matching analysis for each matching parameter determined by the standardized mean differences. c Illustration of propensity scores obtained as described in a for matched (blue: IORT; red: EBRT) and unmatched BM patients (green). BM brain metastasis, EBRT external beam radiation therapy; IORT intraoperative radiotherapy, KPS Karnofsky performance score.
Discussion
IORT following BM resection is an emerging alternative to adjuvant EBRT, but long-term experience and efficiency are yet to be established. Taken together with our previous study [28], this is the first report on IORT for BM that covers both short and long-term clinical FU of a consecutive patient cohort and matches and compares their survival outcomes to those of EBRT.
There is consensus on the beneficial effect of adjuvant RT on local control after BM resection [14, 16, 37]. However, depending on the individual clinical context, it remains controversial which RT sequencing and technique achieves best long-term outcomes at lowest toxicity levels. Despite providing convincing BDFS [38, 39], WBRT was abandoned due to an inferior toxicity profile [15, 40,41,42] in comparison to modern stereotactic RT approaches. Accordingly, previous intracavitary treatment modalities, such as permanent intracerebral radio-isotopic seed implantation, yielded very good LCRs, [43,44,45] yet are prone to induce RN [46, 47]. Moreover, arterial occlusion [48], seed detachment and necessity of subsequent re-surgery could arise. For stereotactic RT, reports on LCR and toxicity differ significantly depending on entities, BM volume and number, but also the fractionation scheme [14, 15, 17, 18, 49]. Besides classical outcome parameters, patient-centered factors such as reduction of hospitalization times, timely treatment access and quality of life have become increasingly important both from patient-centered and socioeconomic view points. This applies in particular to BM patients in a palliative care setting that may suffer from neurological impairment along with a limited life expectancy. Additionally, most patients from our collective were first diagnosed with BM during staging of an extracranial primary tumor. For these patients, swiftness is particularly important, since at time of brain surgery they often still require completion of staging examinations and the initiation of systemic treatment [50]. IORT can expedite these urgent subsequent steps by approximately two to 3 weeks. Furthermore, patients at first diagnosis of metastatic cancer [51], especially with favorable prognostic factors like solitary BM [52], are likely to experience DBP requiring reirradiation to potentially closely located brain structures. The specific physical features of 50 kV IORT provide an increased linear energy transfer and a higher relative biological effectiveness [53] with steep dose gradients allowing both optimized tumoricidal effect and OAR sparing. Thus, patients may benefit from preservation of neurological functions and improved subsequent reirradiation options. The main disadvantage of IORT is a lack of dose modulation options that render certain anatomic conditions challenging. Therefore, it is not surprising that, in line with previous reports [20], most of the BM treated in this cohort were located either craniofrontal or occipital.
Consistent with our previously reported perioperative safety profile [28], we here report a favorable overall RN rate of just 2.9% after IORT. This is an improvement in comparison to adjuvant SRS or FSRT where RN rates typically range between 8% [14] to more than 20% [49], but also to some previous series of IORT patients. While Kahl et al. reported 2.5% [22], Cifarelli et al. noted a RN rate of 7% [19] and Diehl et al. of 11.1% [20]. Of note, the latter also included few patients receiving additional post-surgery stereotactic radiosurgery. In line with previous reports, we found only a comparably low incidence of leptomeningeal spread after IORT [19, 22]. This may be an additional clinical advantage of IORT over other RT techniques that requires further scientific attention. Our observed 1-year LCR of 97.1% compares well with the 94% observed by Cifarelli et al. [19] and outperforms most studies on both adjuvant and definitive SRS or FSRT with rates roughly between 80 to 90% [14, 17, 18, 49, 54,55,56]. Definitive SRS of BM is the primary alternative option to resection when systemic treatment delays are to be avoided. Both effectiveness and safety of single fraction EBRT mainly depend on lesion volume [55]. Compared to SRS only [56], our data indicate a superior LCR and RN rate for IORT of BM > 2 cm, while equally avoiding additional treatment times following surgery.
By matched pair analysis, we demonstrated comparable long-term survival outcomes of EBRT and IORT. The 1 year-survival rate of 57% reported here is also within the range of previous reports for IORT [20, 22]. Meanwhile, despite being marginally different, the matched cohort exhibited outstanding long-term survival. Many of the patients from this cohort surpassed a FU that timewise cannot be achieved yet for the IORT group and, thus, long-term survivors are censored at an earlier time point in the latter. In addition, there are remaining risk factors that could not be adjusted between the groups. While age, CCI, KPS and singularity of BM were considered as matching factors, DS-GPA scores were not. Depending on the tumor entity, this score covers further disease-specific risk factors. However, DS-GPA scores do not qualify for matching analyses as they are not applicable to all tumor entities, nor are they prognostically comparable between different entities [32]. The IORT cohort had a relatively low median DS-GPA score of only 2 and included a total of 25.7% of patients with at least 3 BM. Regardless of these unfavorable prognostic factors, the IORT cohort achieved outstanding local control as well as convincing RN rates in comparison to previous reports, while demonstrating equal long-term outcomes compared to matched EBRT cases.
Limitations
Although the present study had a prospective observational design, its interpretation should take into account several limitations. The most significant limitation is the relatively small sample size of 35 patients, which may impact the generalizability of the findings. Of note, IORT remains a novel treatment option for BM with only very limited data available from comparably sized patient collectives. As an additional methodological measure, using a matched-pair approach could have helped to mitigate some confounding factors when comparing the long-term outcome of patients undergoing EBRT and IORT to BM. However, certain confounding factors, such as different prognostic profiles according to each histology or variable systemic treatment effects, were not regarded. Moreover, since FU MRIs were frequently carried out in local centers using minimized imaging protocols lacking DSC and/or DWI, no reliable data on local control and RN rates were available for the comparative cohort, hence it could not be included in the analysis. Despite these limitations in sample size, the present study may suffice to conceive further large-scale, cross-regional databases to accurately evaluate the safety, feasibility, and efficacy of IORT in the setting of BM surgery. This is the most comprehensive investigation on an IORT patient cohort thus far, incorporating dosimetric aspects, perioperative mortality and RN rate, as well as survival and local control outcomes.
Conclusions
IORT is a timely feasible fast-track approach for complementing surgical BM treatment, with long-term safety and control outcomes comparable to those of adjuvant stereotactic RT. On-going phase II and III studies will soon elucidate the actual role of IORT in this setting.
Data availability
The data presented in this study are available in this article. Further datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cagney DN, Martin AM, Catalano PJ et al (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19:1511–1521. https://doi.org/10.1093/neuonc/nox077
Antonia SJ, Villegas A, Daniel D et al (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379:2342–2350. https://doi.org/10.1056/NEJMoa1809697
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277–1290. https://doi.org/10.1056/NEJMoa1712126
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535–1546. https://doi.org/10.1056/NEJMoa1910836
Modi S, Jacot W, Yamashita T et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54. https://doi.org/10.1007/s11912-011-0203-y
Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177. https://doi.org/10.1093/neuonc/nos152
Yamamoto M, Sato Y, Serizawa T et al (2012) Subclassification of recursive partitioning analysis class ii patients with brain metastases treated radiosurgically. Int J Radiation Oncol Biol Phys 83:1399–1405. https://doi.org/10.1016/j.ijrobp.2011.10.018
Nieder C, Stanisavljevic L, Aanes SG et al (2022) 30-day mortality in patients treated for brain metastases: extracranial causes dominate. Radiat Oncol 17:92. https://doi.org/10.1186/s13014-022-02062-x
Schödel P, Schebesch K-M, Brawanski A, Proescholdt M (2013) Surgical resection of brain metastases—impact on neurological outcome. IJMS 14:8708–8718. https://doi.org/10.3390/ijms14058708
Verhaak E, Gehring K, Hanssens PEJ, Sitskoorn MM (2019) Health-related quality of life of patients with brain metastases selected for stereotactic radiosurgery. J Neurooncol 143:537–546. https://doi.org/10.1007/s11060-019-03186-z
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. JCO 29:134–141. https://doi.org/10.1200/JCO.2010.30.1655
Lehrer EJ, Peterson JL, Zaorsky NG et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiation Oncol Biol Phys 103:618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Eitz KA, Lo SS, Soliman H et al (2020) Multi-institutional analysis of prognostic factors and outcomes after hypofractionated stereotactic radiotherapy to the resection cavity in patients with brain metastases. JAMA Oncol 6:1901. https://doi.org/10.1001/jamaoncol.2020.4630
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060. https://doi.org/10.1016/S1470-2045(17)30441-2
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
Jhaveri J, Chowdhary M, Zhang X et al (2019) Does size matter? Investigating the optimal planning target volume margin for postoperative stereotactic radiosurgery to resected brain metastases. J Neurosurg 130:797–803. https://doi.org/10.3171/2017.9.JNS171735
Layer JP, Layer K, Sarria GR et al (2023) Five-Fraction stereotactic radiotherapy for brain metastases—a retrospective analysis. Curr Oncol 30:1300–1313. https://doi.org/10.3390/curroncol30020101
Cifarelli CP, Brehmer S, Vargo JA et al (2019) Intraoperative radiotherapy (IORT) for surgically resected brain metastases: outcome analysis of an international cooperative study. J Neurooncol 145:391–397. https://doi.org/10.1007/s11060-019-03309-6
Diehl CD, Pigorsch SU, Gempt J et al (2022) Low-energy X-Ray Intraoperative radiation therapy (Lex-IORT) for resected brain metastases: a single-institution experience. Cancers 15:14. https://doi.org/10.3390/cancers15010014
Krauss P, Steininger K, Motov S et al (2022) Resection of supratentorial brain metastases with intraoperative radiotherapy Is it safe? Analysis and experiences of a single center cohort. Front Surg 9:1071804. https://doi.org/10.3389/fsurg.2022.1071804
Kahl K-H, Balagiannis N, Höck M et al (2021) Intraoperative radiotherapy with low-energy x-rays after neurosurgical resection of brain metastases-an Augsburg University Medical center experience. Strahlenther Onkol 197:1124–1130. https://doi.org/10.1007/s00066-021-01831-z
Sarria GR, Smalec Z, Muedder T et al (2021) Dosimetric comparison of upfront boosting with stereotactic radiosurgery versus intraoperative radiotherapy for glioblastoma. Front Oncol 11:759873. https://doi.org/10.3389/fonc.2021.759873
Dahshan BA, Weir JS, Bice RP et al (2021) Dose homogeneity analysis of adjuvant radiation treatment in surgically resected brain metastases: comparison of IORT, SRS, and IMRT indices. Brachytherapy 20:426–432. https://doi.org/10.1016/j.brachy.2020.11.004
Giordano FA, Brehmer S, Mürle B et al (2019) Intraoperative radiotherapy in newly diagnosed glioblastoma (INTRAGO): an open-label, dose-escalation phase I/II trial. Neurosurgery 84:41–49. https://doi.org/10.1093/neuros/nyy018
Sarria GR, Sperk E, Han X et al (2020) Intraoperative radiotherapy for glioblastoma: an international pooled analysis. Radiother Oncol 142:162–167. https://doi.org/10.1016/j.radonc.2019.09.023
Herskind C, Ma L, Liu Q et al (2017) Biology of high single doses of IORT: RBE, 5 R’s, and other biological aspects. Radiat Oncol 12:24. https://doi.org/10.1186/s13014-016-0750-3
Hamed M, Potthoff A-L, Layer JP et al (2022) Benchmarking safety indicators of surgical treatment of brain metastases combined with intraoperative radiotherapy: results of prospective observational study with comparative matched-pair analysis. Cancers 14:1515. https://doi.org/10.3390/cancers14061515
Schäfer N, Bumes E, Eberle F et al (2021) Implementation, relevance, and virtual adaptation of neuro-oncological tumor boards during the COVID-19 pandemic: a nationwide provider survey. J Neurooncol 153:479–485. https://doi.org/10.1007/s11060-021-03784-w
Hamed M, Potthoff A-L, Heimann M et al (2023) Survival in patients with surgically treated brain metastases: does infratentorial location matter? Neurosurg Rev 46:80. https://doi.org/10.1007/s10143-023-01986-6
Péus D, Newcomb N, Hofer S (2013) Appraisal of the karnofsky performance status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak 13:72. https://doi.org/10.1186/1472-6947-13-72
Sperduto PW, Kased N, Roberge D et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. JCO 30:419–425. https://doi.org/10.1200/JCO.2011.38.0527
Marks LB, Yorke ED, Jackson A et al (2010) Use of normal tissue complication probability models in the clinic. Int J Radiation Oncol Biol Phys 76:S10–S19. https://doi.org/10.1016/j.ijrobp.2009.07.1754
Heimann M, Schäfer N, Bode C et al (2021) Outcome of elderly patients with surgically treated brain metastases. Front Oncol 11:713965. https://doi.org/10.3389/fonc.2021.713965
Schneider M, Heimann M, Schaub C et al (2020) Comorbidity burden and presence of multiple intracranial lesions are associated with adverse events after surgical treatment of patients with brain metastases. Cancers 12:3209. https://doi.org/10.3390/cancers12113209
Ilic I, Potthoff A-L, Borger V et al (2022) Bone Mineral density as an individual prognostic biomarker in patients with surgically-treated brain metastasis from lung cancer (NSCLC). Cancers 14:4633. https://doi.org/10.3390/cancers14194633
Patchell RA, Tibbs PA, Regine WF et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489. https://doi.org/10.1001/jama.280.17.1485
Sahgal A, Aoyama H, Kocher M et al (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiation Oncol Biol Phys 91:710–717. https://doi.org/10.1016/j.ijrobp.2014.10.024
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401. https://doi.org/10.1001/jama.2016.9839
Soffietti R, Kocher M, Abacioglu UM et al (2013) A European organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 31:65–72. https://doi.org/10.1200/JCO.2011.41.0639
Mulvenna P, Nankivell M, Barton R et al (2016) Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 388:2004–2014. https://doi.org/10.1016/S0140-6736(16)30825-X
Hong AM, Fogarty GB, Dolven-Jacobsen K et al (2019) Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: a multicenter, randomized phase III trial. J Clin Oncol 37:3132–3141. https://doi.org/10.1200/JCO.19.01414
Wernicke AG, Hirschfeld CB, Smith AW et al (2017) Clinical outcomes of large brain metastases treated with neurosurgical resection and intraoperative cesium-131 brachytherapy: results of a prospective trial. Int J Radiat Oncol Biol Phys 98:1059–1068. https://doi.org/10.1016/j.ijrobp.2017.03.044
Raleigh DR, Seymour ZA, Tomlin B et al (2017) Resection and brain brachytherapy with permanent iodine-125 sources for brain metastasis. J Neurosurg 126:1749–1755. https://doi.org/10.3171/2016.4.JNS152530
Yang L, Wang C, Zhang W et al (2022) Iodine-125 brachytherapy treatment for newly diagnosed brain metastasis in non-small cell lung cancer: a biocentric analysis. Front Oncol 12:1005876. https://doi.org/10.3389/fonc.2022.1005876
Wowra B, Schmitt HP, Sturm V (1989) Incidence of late radiation necrosis with transient mass effect after interstitial low dose rate radiotherapy for cerebral gliomas. Acta Neurochir (Wien) 99:104–108. https://doi.org/10.1007/BF01402316
Huang K, Sneed PK, Kunwar S et al (2009) Surgical resection and permanent iodine-125 brachytherapy for brain metastases. J Neurooncol 91:83–93. https://doi.org/10.1007/s11060-008-9686-2
Bernstein M, Lumley M, Davidson G et al (1993) Intracranial arterial occlusion associated with high-activity iodine-125 brachytherapy for glioblastoma. J Neuro-Oncol 17:253–260. https://doi.org/10.1007/BF01049980
Doré M, Martin S, Delpon G et al (2017) Stereotactic radiotherapy following surgery for brain metastasis: predictive factors for local control and radionecrosis. Cancer/Radiothérapie 21:4–9. https://doi.org/10.1016/j.canrad.2016.06.010
Potthoff A-L, Heimann M, Lehmann F et al (2023) Survival after resection of brain metastasis: impact of synchronous versus metachronous metastatic disease. J Neurooncol. https://doi.org/10.1007/s11060-023-04242-5
Lutterbach J, Cyron D, Henne K, Ostertag CB (2008) Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery 62(Suppl 2):776–784. https://doi.org/10.1227/01.neu.0000316281.07124.ea
Sawrie SM, Guthrie BL, Spencer SA et al (2008) Predictors of distant brain recurrence for patients with newly diagnosed brain metastases treated with stereotactic radiosurgery alone. Int J Radiat Oncol Biol Phys 70:181–186. https://doi.org/10.1016/j.ijrobp.2007.05.084
Liu Q, Schneider F, Ma L et al (2013) Relative biologic effectiveness (RBE) of 50 kV X-rays measured in a phantom for intraoperative tumor-bed irradiation. Int J Radiat Oncol Biol Phys 85:1127–1133. https://doi.org/10.1016/j.ijrobp.2012.08.005
Lehrer EJ, Ahluwalia MS, Gurewitz J et al (2022) Imaging-defined necrosis after treatment with single-fraction stereotactic radiosurgery and immune checkpoint inhibitors and its potential association with improved outcomes in patients with brain metastases: an international multicenter study of 697 patients. J Neurosurg. https://doi.org/10.3171/2022.7.JNS22752
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48. https://doi.org/10.1186/1748-717X-6-48
Minniti G, Scaringi C, Paolini S et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Acknowledgements
We thank Katja Klever and Monika Brüggemann for their support in patient follow-up and the medical team of Helmut Forstbauer for aiding in data acquisition.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that the article content was composed in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest. JPL was supported by a grant from Novartis Stiftung für therapeutische Forschung.
Author information
Authors and Affiliations
Contributions
This study was conceptualized by JPL, MS and LCS. Surgery was performed by MH, ALP, VB, HV and MS. IORT was planned and performed by JPL, CSD, KL, DS, DK, MK, FK, MG, JAH, SG, FAG and LCS. Patient follow-up was conducted by JPL, MH, APL, CSD, KL, DS, DK, MK, TZ, LLF, VB, FCS, JW, NS, HF, FAG, UH, HV, MS, LCS. Material preparation and data collection were performed by JPL, ALP and MS. Analysis was performed by JPL and ALP. MH, FAG, HV, MS and LCS provided funding and resources. The first draft of the manuscript was written by JPL. GRS, MS and LCS reviewed and edited the data and manuscript. All authors commented on previous versions and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
J.P.L reports stocks and travel expenses from TME/NOXXON Pharma AG; stocks and honoraria from Siemens Healthineers and stocks from Bayer AG and BioNTech AG. F.A.G. reports research grants and travel expenses from ELEKTA AB and Varian Medical Systems Inc.; grants, stocks, travel expenses and honoraria from TME/NOXXON Pharma AG; research grants, travel expenses and honoraria from Carl Zeiss Meditec AG; travel expenses and honoraria from Bristol-Myers Squibb, Cureteq AG, Guerbet SA, Roche Pharma AG, MSD Sharp and Dohme GmbH and AstraZeneca GmbH; non-financial support from Oncare GmbH and Opasca GmbH. G.R.S. reports personal fees and travel expenses from Carl Zeiss Meditec AG, personal fees from Roche Pharma AG, personal fees from MedWave Clinical Trials and travel expenses from Guerbet SA, not related to this work. U. H. reports advisory Board and/or lecture honoraria from Bayer AG and Medac GmbH.
Ethics approval
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital Bonn (Approval Number: 018/21 and 057/22). Informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Layer, J.P., Hamed, M., Potthoff, AL. et al. Outcome assessment of intraoperative radiotherapy for brain metastases: results of a prospective observational study with comparative matched-pair analysis. J Neurooncol 164, 107–116 (2023). https://doi.org/10.1007/s11060-023-04380-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04380-w