Abstract
Microalgae can form renewable feedstock for many sectors. However, most microalgae commercially cultivated are difficult to grow in colder climates. To overcome this problem one could cultivate cold tolerant algae. One such alga is the red marine alga Porphyridium purpureum. It is currently often cultivated in bag systems in limited volumes. In this study we cultivated P. purpureum at pilot-scale in horizontal tubular reactors in a greenhouse. P. purpureum was grown in batch mode in a total volume of either 350, 500, 1000 or 1500 L. A total of 28 batches, spread over two years, were cultivated reaching a maximum dry weight of 1.94 g L−1. The growth was continuously monitored to calculate the productivities and growth rates. The P and N concentrations were measured frequently during four batches to calculate the P and N usage per gram biomass. To calculate CO2 fixation efficiencies the total CO2 consumption was monitored during 6 of the 28 batches. A maximum productivity and growth rate of respectively 0.195 g L−1 day−1 and 0.233 day−1 were achieved while the P and N usage varied from 3.2–6.19 and 14.5–36.6 mg g−1 biomass for P and N, respectively. Growth was supported between 10 °C and 30 °C, while temperatures above 34—35 °C proved to be detrimental. The CO2 efficiencies differed among the 6 tested periods with the highest utilization efficiency being 13.5% and the lowest a mere 1.5%. Further large-scale, continuous, tests would be needed to fully optimize the cultivation of P. purpureum in larger volumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consisting of several thousand species (Guiry 2012), microalgae form a large and diverse assemblage of single-celled eukaryotic organisms that can be found in a wide variety of habitats. Using sunlight as energy and CO2 as a carbon source they can produce biomass through photosynthesis (Benedetti et al. 2018; Vecchi et al. 2020), a process they can perform 10 times more efficiently than terrestrial plants. In combination with fast growth rates, they form a promising valuable source of renewable feedstock for multiple applications (Singh and Ahluwalia 2013; Benedetti et al. 2018; Khan et al. 2018). While the main focus for microalgal production in the last decades was energy based, more focus is currently also put on the production of biomass for feed and food, nutraceutical and biomedical applications (Fuentes-Grünewald et al. 2015; Bhalamurugan et al. 2018).
Due to its vast potential, the cultivation of microalgae has received a lot of attention in recent years, yet large-scale production of microalgae remains largely underexplored. Only a select few species are currently produced on a commercial scale (Richmond 2000; Singh and Ahluwalia 2013; Benedetti et al. 2018; Khan et al. 2018; Dolganyuk et al. 2020; Araújo et al. 2021). The legislative hurdles, high production cost, and most research being done at well-controlled laboratory scale form bottlenecks for a swifter upscaling of microalgae cultivation (Fernández et al. 1998; Tredici 2010; Quinn et al. 2011; Pérez-López et al. 2017; Araújo et al. 2021; Latsos et al. 2022).
Porphyridium purpureum (formerly P. cruentum) is one of the select few microalgal species already cultivated commercially (Gaignard et al. 2019; Li et al. 2019a; Araújo et al. 2021). It is a unicellular eukaryotic marine red algal species belonging to the Rhodophyta (Sato et al. 2017; Gaignard et al. 2019; Li et al. 2019a). Being a marine species, it can be cultivated in seawater and thus be more sustainable as no or less fresh water or nutrients are needed (Moomaw et al. 2017; Merlo et al. 2021). However, currently often artificial seawater is used (Gaignard et al. 2019). Furthermore, it can still be cultivated at lower temperatures (Durmaz et al. 2007; Guihéneuf and Stengel 2015), making it an interesting species for countries with colder climates. It lacks a cell wall and produces extracellular polysaccharides (EPS) surrounding its cell (Gaignard et al. 2019). Aside from EPS it produces and accumulates several valuable bioactive substances such as unsaturated fatty acids and phycobiliproteins (e.g., phycoerythrin) (Gaignard et al. 2019; Li et al. 2019a; Bayu et al. 2022). Research on P. purpureum dates back to the 1950s (Brody and Emerson 1959; Golueke and Oswald 1962) and recently more in-depth studies have started because of its potential economic value (Fuentes-Grünewald et al. 2015; Sato et al. 2017). Due to the aforementioned, more (mainly laboratory-scale) research has been published on Porphyridium spp. and it is on its way to become a model rhodophyte (Li and Bock 2018). While the genetic exploitation of P. purpureum is still in its initial stage (Li et al. 2019a), its genome has been sequenced and genetic transformation tools have been developed (Perrineau et al. 2015; Li and Bock 2018; Li et al. 2019a).
At larger-scale, even though already being produced commercially, its full potential remains underexplored (Li et al. 2019b; Yin et al. 2022). Current commercial cultivation, mainly for EPS production, occurs mostly in batch mode in short lived, single use bags prone to biofouling. The cultivation volumes are often also small (60–80 L) (Tredici 2003; Fuentes-Grünewald et al. 2015). Research done in the laboratory in well-controlled small volumes makes it difficult to extrapolate to larger-scale cultivation (Tredici 2010; Quinn et al. 2011; Latsos et al. 2022). Specifically for Porphyridium spp., only a handful of studies describe its cultivation in a larger-scale set-up (Gaignard et al. 2019; Li et al. 2019a) of which one study described the cultivation in a total volume of 7000 L (Chaumont et al 1988; Chaumont 1993). To generate more larger-scale cultivation data outside the well controllable lab conditions and gain more insight on long-term cultivation in a tubular photobioreactor using larger cultivation volumes, we performed multiple and long-term pilot-scale cultivation tests in a total volume of up to 1500 L in a tubular photobioreactor placed inside a greenhouse located in Geel, Belgium. Furthermore, the growth was continuously monitored by turbidity measurement to determine the growth pattern.
Materials and methods
Microalga strain and culture conditions
Porphyridium purpureum (SAG 1380-1C) was purchased from SAG (Department Experimental Phycology and Culture Collection of Algae, University of Göttingen, Germany). The culture was maintained in the laboratory in brackish water medium based on the SAG brackish water medium recipe (version 10.2008). The medium was sterilized by autoclaving (121 °C for 20 min). Porphyridium purpureum was kept in 250 mL-Erlenmeyers on an orbital shaker at 90 rpm with 70 µmol photons m−2 s−1 light exposure (cool-white fluorescent) at 22 °C (± 0.2 SD) under a 16/8 h day/night regime. Cultures were subsequently transferred to aerated 1, 2 and 40 L recipients for upscaling. During upscaling autoclaved medium and ambient air was used for aeration and mixing. No extra CO2 was provided during upscaling. For pilot-scale cultivation in the photobioreactors the same brackish medium, sterilized by filtration (0.2 μm), was used. The medium had the following composition: 252 mg L−1 HNO3, 22 mg L−1 H3PO4, 248 mg L−1 KOH, 6.3 mg L−1 Fe-DTPA, 42 pg L−1 CuSO4.5H2O, 2.8 µg L−1 ZnSO4, 7.2 µg L−1 MnSO4, 4.3 µg L−1 Na2MoO4, 40.1 µg L−1 Na2B4O7, 0.2 g L−1 NaHCO3 and 3.5 g L−1 MgSO4.7H2O, 11.8 g L−1 NaCl and 0.315 g L−1 CaCl2.2H2O. Furthermore 0.5 mg L−1 Vitamin B12 was added during each step of the scaling-up. For cultivation in the photobioreactors Vitamin B12 was added at the initial start of the culture, but not afterwards. During cultivation in the photobioreactors HNO3 and H3PO4 were added to the culture when needed, based on regular measurement of their concentrations. For medium preparation for the pilot-scale cultivation a central autonomously computer control unit was used and the medium was fed to the cultures by a feed supply unit (FertiMiX 600).
Determination of microalgal growth
For continuous monitoring of the growth at pilot-scale, a continuous online turbidity monitoring system (Georg Fischer Signet 4150 turbimeter 0–1000 Nephelometric Turbidity Unit, NTU) was used. The turbidity was measured every 30 min and the corresponding dry weight could be calculated using the correlation previously determined (Thoré et al. 2021b):
with x being the turbidity (Thoré et al. 2021b). The dry weight was furthermore regularly manually measured, specifically at the start and end of each batch and during the cultivation. Both measurements were combined to follow the growth. For dry weight measurements samples (5 mL per sample) were filtered on glass microfiber membranes (0.45 µm), dried at 70 °C for 24 h and transferred to a desiccator before weighing. Regular microscopic evaluation during the cultivation was also performed to check the purity of the culture. Non-contaminated cultures were defined as cultures free of excessive presence of bacteria, fungi, grazers or other algae. The pilot-scale cultures however were not axenic, as the presence of minute traces of other microorganisms is nearly unavoidable in large volume pilot-scale setups such as the ones used in this study.
The growth was evaluated by calculating the growth rate (μ) based on turbidity measurements and dry weight measurements, volumetric productivity and doubling time based on the start and end concentrations of each batch cultivated in the photobioreactor.
Horizontal tubular multilayer photobioreactors
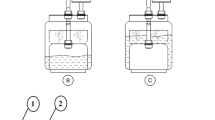
Three tubular multilayer photobioreactors with volumes of respectively 350 L, designated reactor 3 (R3) and reactor 4 (R4) and 1500 L, designated reactor 1 (R1), were used for cultivation of P. purpureum. The 1500 L reactor consists of 3 smaller units of 500 L connected to each other with valves (Fig. 1). Depending on the experiment, either a 500 L, 1000 L or 1500 L configuration was used. All reactors are located in a greenhouse in Geel, Belgium and are part of the pilot plant of the EU EFRO-project “Sunbuilt”. The photobioreactors consist of transparent unplasticized polyvinylchloride (PVC-u) tubes with an external diameter of 5 cm. To avoid shading of lower located tubes the photobioreactors were constructed in a triangle-like configuration (Fig. 1).
The photobioreactors used to perform the growth experiments with P. purpureum. A. The left photobioreactor is a 350 L reactor of which 2 separate reactors were used (R3 and R4). B. The 1500 L reactor which consists of 3 (1, 2 and 3) units of 500 L that can be used separately or together (either 500, 1000 or 1500 L total volume) is shown on the right
Operating procedure of the photobioreactor
Porphyridium purpureum was successfully cultivated in batch mode for a total of 28 batches between January 2018 and January 2020. In total 14 batches were cultivated in the 350 L reactors, while 12 batches were grown in the 1500 L reactor. The remaining 2 batches were cultivated in a total volume of 500 and 1000 L respectively (Fig. 2). The growth tests were performed in non-nutrient limiting conditions and partial harvests (50 to 80%) occurred at the end of each batch. Cultivation continued with the remaining culture and fresh medium (same volume fresh medium was added as the harvested volume). The growth tests took place in the spring, winter and fall periods. Two batches, not included in the list of 28 batches, were cultivated during the summer (both in 2018 and 2019) but crashed after a few days due to temperatures reaching 35 °C and higher. Since temperatures of 35 °C during the summer are no exception, no further cultivation attempts took place during the summer periods. A third batch, not mentioned in the list of 28, was a cultivation attempt in October 2020. However, the culture halted growth after only 10 days.
A centrifugal pump (900 rpm) was used to circulate the medium and filter-sterilized (0.1 μm) ambient air was injected in the lowest tube at a continuous rate creating a turbulent flow for mixing. Since P. purpureum is generally cultivated at a pH between 7.5 and 8, the working pH was set at a value between 7.5 and 8.5 (Singh et al. 2000; You and Barnett 2004). However, a few batches were also cultivated at a pH setpoint of 9 or 10. The pH was continuously measured and maintained by the injection of CO2 on demand. Filter-sterilized (0.1 μm) CO2 was injected in the airflow, with a maximum flow of 885.9 mL min−1 (350 L reactor) or 5600 mL min−1 (1500 L reactor). To have an indication of the CO2 usage during the growth of P. purpureum, the total CO2 injected in the 350 L photobioreactor was continuously measured during 6 batches, cultivated between 5 October 2018 and 12 December 2018, by an in-flow mass flow meter/controller (Bronkhorst, the Netherlands). See also supplemental Fig. 1 for a more in-depth overview of the configuration of the reactors used in this study.
The ambient temperature inside the greenhouse was monitored (Ektron III-C, Hortiplan) continuously. To prevent an excessive rise in temperature in the greenhouse, foggers inside the greenhouse and sprinklers on the roof were turned on automatically when the temperature reached 23 °C. A gas heater (HHB-100A-230 V, Holland Heaters) was used to maintain a minimal temperature of 10 °C. Aside from the heater, an energy screen was closed between 16.00 and 8.00 during the colder periods to keep the heat in the greenhouse. The photosynthetically active radiation (PAR) was measured continuously with a PAR sensor (LI-COR LI-190R Quantum sensor) installed inside the greenhouse, on top of the reactor. A solar irradiance meter (LP02-TR pyranometer) was installed outside the greenhouse to measure total solar radiation. At a measured value of 400 W m−2, a sunscreen inside the greenhouse was automatically partially closed to reduce irradiation by 20–30%.
To provide more light, artificial lighting was provided during shorter days (Philips TL-D 58W 865; approximately 504 mmol photons m−2 day−1) to lengthen the daytime to approximately 14–16 h. Control, logging and steering was done automatically by computer (MultiMa series III, Hortiplan).
Determination of nitrogen and phosphorus in the culture medium
To determine the nitrogen and phosphorus usage of P. purpureum at pilot-scale 50 mL samples were taken at regular intervals to determine the usage of N and P. Based on the remaining N and P in the samples, extra N and P was added to the photobioreactors to provide non-nutrient limiting growth. Samples were subsequently centrifuged for 10 min at 1500 ×g at 20 °C and either directly analyzed or frozen before analysis.
Remaining nitrogen content was analyzed as nitrate by ionic chromatography (Metrohm Eco IC using a Metrosep A Supp 17–250/4.0 column) and a 6 mM Na2CO3 buffer. Samples were pretreated by filtering through 0.45 µm and 0.20 µm disposable PET-filters (NBN EN ISO 10304–1). The obtained concentration of nitrate was recalculated to nitrogen concentration based on molecular weights.
Phosphorus content in the medium was determined by ICP-OES (Optima 4300 DV, Perkin Elmer Instruments). Samples were centrifuged for 10 min at 3000 ×g to remove algal biomass. A 9 mL sample of the algae medium was taken and acidified with 1 mL of concentrated nitric acid (65%). The sample was homogenized and phosphorus was measured by ICP-OES at 214 nm.
Calculations
For each batch the volumetric biomass productivity Pv (g L−1 day−1) was calculated using Eq. 1:
with Cs and Ce the start and end biomass concentration (g L−1), respectively, and tc the cultivation time (days) for each specific growth period (Islam et al. 2021).
To be able to calculate the biomass yield on light for each batch the areal productivity (Pa) was also calculated. This was done by the formula (Eq. 2):
with V the volume of the reactor, Cs and Ce the start and end biomass concentration (g L−1), respectively, Ar the area occupied by the reactor used and tc the cultivation time (days) for each specific batch (Islam et al. 2021). The 350 L reactor had a surface area of 14 m2 while the 1500 L reactor had a total surface area of 120 m2 (for each unit of 500 L a surface area of 40 m2 was used, surface area was defined as the surface occupied by the PBR, including the degasser).
The following adapted Eq. (3), using the areal instead of the volumetric productivity (Molina Grima et al. 1997), was used to calculate the biomass yield on light (PL, g mol−1 photons) for each specific batch:
with Pa the areal productivity and PARc the average PAR (mol photons m−2 day−1) received during each day (light-period) of that specific batch.
The specific growth rate (day−1) was calculated for each batch using Eq. 4:
with Ne and Ns the biomass (g) at, respectively, the end and start of each period and tc being the total number of days in the specific period (Lee and Shen 2003).
The doubling time (days) was calculated as (Eq. 5):
with µ being the calculated specific growth rate (Islam et al. 2021). For a doubling time in hours, Td was multiplied by 24.
During four batches, the nitrogen (N) and phosphorus (P) content were measured frequently to monitor the N and P usage by the algae. The used N and P was then calculated using Eqs. 6 and 7:
and
with NU the nitrogen used, Ns the nitrogen content at the start, Ne the nitrogen content at the end, PU the phosphorus used, Ps the P content at the start and Pe the P content at the end of the growth experiment. To calculate the used N and P per gram biomass per experiment, the used P and N was divided by the biomass (g) produced during the specific experiment. The N/P ratio was calculated by dividing the consumed N (mg L−1) by the consumed P (mg L−1). For the other growth periods, the N and P content were measured at regular intervals and extra N and P was added to the culture when needed.
To investigate the estimated consumption of CO2 by P. purpureum the total CO2 injected was measured online and continuously by a mass flow meter (In-Flow mass flow meter/controller Bronkhorst, the Netherlands) for 5 batches in the 350 L photobioreactors. The theoretical total CO2 fixation (CO2th, g) by the algae for each batch was calculated as in Eq. 8:
with Cs and Ce the start and end biomass concentration (g L−1), \({m}_{{CO}_{2}}{, m}_{C}\) being the molar mass of respectively CO2 and C, Vr the volume of the reactor and 0.43 a percentage based on the carbon content (Moraes et al. 2016). Often the factor 0.5 is used for the carbon content in microalgae (Goldman et al. 1971; Sánchez Mirón et al. 2003; Tang et al. 2011). However, using this assumption might lead to under- or overestimation as several authors already showed that the carbon content can deviate substantially from the 0.5 factor (Goldman et al. 1971; Ahlgren et al. 1992; Reitan et al. 2021). Since we did not measure the specific carbon content, we used a factor of 0.43 which was previously described for P. purpureum (UTEX 161) (Lee and Bazin 1991).
The CO2 fixation rate CO2fr was calculated using Eq. 9:
with 1.58 derived from multiplying the average carbon content (43%) per unit biomass with the CO2 carbon ratio (44/12) (Ho et al. 2017).
Equation 10 was used to have an idea about the estimated CO2 uptake efficiency. The calculated CO2th, was divided by the total CO2 (CO2t, g) injected (influent CO2) and multiplied by 100 to know the utilization efficiency percentage (U%):
The above formula is a simplified version of the formula: \(\frac{\mathrm{Influent}\;\mathrm{of}\;{\mathrm{CO}}_2-\mathrm{effluent}\;{\mathrm{CO}}_2}{\mathrm{influent}\;{\mathrm{CO}}_2}100\) (Klinthong et al. 2015) in which the numerator is replaced by the theoretical total CO2 fixation since the photobioreactor had no measurement of CO2 in the outgoing gases. The total loss of CO2 (CO2tL) was calculated by subtracting CO2th from CO2t giving Eq. 11:
Statistical analysis
To compare the differences in temperatures, received PAR and daily biomass production between the 28 batches a one-way analysis of variance (ANOVA) with post hoc Tukey HSD with a significance level of 0.05 was performed. Normality was tested with a Shapiro–Wilk test. Data are given as mean ± SD.
Results
Growth of P. purpureum and N , P usage at pilot-scale
To move beyond well-controlled laboratory-studies we cultivated P. purpureum at pilot-scale volumes up to a total of 1500 L. In total 28 batches were cultivated and harvested over a period of two years (Fig. 3).
Next to the 28 successful batches, three failed experiments were observed. The first two were cultivations attempted during the summer periods in 2018 and 2019 (23 August-2 September 2018 and 24–31 August 2019) that failed due to too hot temperatures. A third failed attempt was in October 2020. The growth of the culture halted after approximately 10 days. The reason for this third failed cultivation is unclear.
The two longest non-stop growth periods had a span of 3 to 4 months, from September 2018 to February 2019 (using multiple reactors) and from October 2019 to January 2020 (using 1 reactor), in which respectively 10 and 7 batches were harvested. Longer cultivation periods however came with an increased risk of biofilm formation and contamination in the culture (Fig. 4). Data used in this study was from non-contaminated cultures as cultivation was halted once contamination was clearly prevalent. For observational use, some batches were kept longer to observe biofilm formation.
A. biofilm formation in the photobioreactor tubes (tube has been rotated for a better view on the biofilm formation, the upper part seen here is the bottom part in real life after 4 months of non-stop cultivation). B. Excessive contamination (green cells) in the P. pupureum culture (red cells). Biofilm formation and contamination occurred after prolonged periods
Figure 5 shows the growth pattern of P. purpureum. In general, the NTU and dry weight increased steadily until a (partial) harvest took place to dilute the culture and start a new batch. On a daily basis, the NTU started to increase from sunrise until sunset. During the dark period the NTU decreased (Fig. 5).
A maximum dry weight of 1.94 g L−1 was reached during our experiments for several batches. Table 1 shows for each batch the growth rate, volumetric productivity, yield on light and doubling time, all calculated based on the initial and final dry weights of each batch. The average daily amount of light (PAR) and average daily temperatures during each batch was also monitored. The extra light (504 mmol m−2 day −1) provided during shorter days is incorporated in the PAR values given in Table 1.
The highest growth rate, productivity and lowest doubling time obtained were respectively 0.233 day−1, 0.195 g L−1 day−1 and 2.97 days. Over the 2-year span an average growth rate, volumetric productivity and doubling time of respectively 0.117 ± 0.047 day−1; 0.084 ± 0.044 g L−1 day−1 and 7.01 ± 3.17 day was obtained. The average PAR light received and average temperature were 45.77 ± 20.6 µmol photons m−2 s−1 and 18.5 ± 2.7 °C respectively.
To compare the 28 batches statistically a one-way analysis of variance was performed to compare the daily growth rates, daily average temperatures and daily average PAR values during each batch (Supplemental Tables 2 and 3). While the daily temperature and daily PAR measured during the 28 batches differed significantly between many batches (Table S3), the daily growth rates however were less often statistically significant different between batches (Table S2). No clear correlation between temperature and growth rate or doubling time could be observed, nor for the average PAR received and the growth rate or doubling time (Fig. S2). When looking at the biomass yield on light, several batches cultivated during the fall and winter, with lower average PAR, achieved high yields on light while the yield on light during periods with higher average PAR intensities was often lower (Table 1).
To estimate the N and P used by P. purpureum at pilot-scale, samples were taken frequently during 4 time periods (2 periods for the 350 L reactor and 2 periods for the 1500 L reactor) to calculate the total N and P used (Table 2).
Table 2 shows the P used stayed between 3.2 and 6.19 mg per g biomass cultivated while the algae used between 14.5 and 36.6 mg N per g biomass. The reason for the differences, especially for N usage, is unclear. The observed N/P ratios shows that the algae use the N and P nutrients effectively as we used a 5/1 ratio for our medium composition.
CO2 utilization efficiency at pilot-scale
The total CO2 injected into the photobioreactors (350 L reactors) was monitored during 6 periods in 2018 to calculate the CO2 utilization efficiency (Table 3).
During the period 9–15 October the utilization efficiency was the highest with 30.9% in R4 at pH 8. Yet, during the same period an efficiency of only 5.1% was reached in the second photobioreactor used (R3) at pH 8.5. The second highest efficiency, 12.2%, was reached in R3 during the period 15–24 October, pH setpoint 8.3. In the same period (R4, pH setpoint 7.5) an efficiency was reached of 10.4%. Two more tests at pH 7.5 were conducted (31 Oct-6 Nov and 16 Nov.-6 Dec) and they only had efficiencies of 5.8 and 7.6% respectively. The highest CO2 efficiencies were achieved at a pH of 8 or 8.3, while a lower (7.5) or higher (8.5) pH setpoint resulted in lower efficiencies.
Discussion
Growth of P. purpureum and N, P usage at pilot-scale
As with most microalgae, the majority of the published work on P. purpureum is done at laboratory-scale (Gaignard et al. 2019; Li et al. 2019a). However, extrapolating of laboratory-scale data to pilot-scale or industrial-scale is arduous (Fernández et al. 1998; Pérez-López et al. 2017) and more large-scale experiments are needed (Borowitzka and Vonshak 2017). One study on P. purpureum that tested both laboratory and larger scale conditions reported biomass productions at larger scale that were 3.8 to 4.6 times lower compared to the laboratory-scale test (Castro-Varela et al. 2021). In general, it is difficult to compare results between studies due to a lack of pilot-scale studies and due to differences in cultivation methods, culture system used and environmental conditions (Borowitzka and Vonshak 2017). Specifically for P. purpureum the scarcity of literature on large-scale P. purpureum cultivation makes it very challenging to compare our data with what others reported for Porphyridium spp. Currently only a few studies describe the cultivation of P. purpureum in larger volumes (> 200 L working volume) in tubular reactors (Gudin and Chaumont 1991; Chaumont 1993; Rebolloso Fuentes et al. 1999; Fuentes-Grünewald et al. 2015; Castro-Varela et al. 2021; Yin et al. 2022). Of these studies, one was limited to a 3-day trial in a 220 L reactor (Rebolloso Fuentes et al. 1999), while a second one was limited to a 11-day experiment in a 700 L reactor (Yin et al. 2022) and a third one was limited to 14 days and no CO2 was provided during the cultivation (Castro-Varela et al. 2021) making it hard to compare batches or estimate longer term cultivation or environmental influences. The fourth study describes the cultivation during a 30–50-day period in two 600 L reactors (Fuentes-Grünewald et al. 2015), while the fifth study was performed in a 7000 L reactor and mentioned a steady state cultivation period for up to 2 months (Gudin and Chaumont 1991; Chaumont 1993). To investigate the further upscaling of P. purpureum we performed several batch cultures up to a total volume of 1500 L (Table 1) during a two-year period from 2018 to 2020 (Fig. 3).
During our two-year cultivation averages of volumetric productivity, growth rate and a maximum dry weight of respectively 0.084 ± 0.044 g L−1 day−1; 0.117 ± 0.047 day−1 and 1.94 g L−1 were obtained. Table 4 shows the results obtained in this study compared to the five other pilot-scale studies we found in the literature and the results of the Arad group who used the standard sleeves.
Rebolloso Fuentes et al. (1999) reached a maximum dry weight of 3.5 g L−1. They reported an average productivity of 1.76 g L−1 day−1 with growth rates of day 1, 2 and 3 of respectively 0.0049, 0.0075 and 0.0107 h−1. Their study was however limited to a 3-day growth experiment (Rebolloso Fuentes et al. 1999) and potentially not indicative for longer cultivation periods. Fuentes-Grünewald et al. (2015) reached a maximum dry weight of 1.04 g L−1 and reported average productivities of 0.027 g L−1 day−1 (batch mode) and 0.047 g L−1 day−1 (semi-continuous) with growth rates of 0.26 day−1 and 0.27 day−1, respectively (Fuentes-Grünewald et al. 2015). Yin et al. (2022) did not specify average productivities nor growth rates but reached a maximum dry weight of 0.85 g L−1 at day 9 (Yin et al. 2022). Castro-Varela et al. (2021) performed two larger scale experiments, one in a 350 L PBR and one on a 700 L open pond system reaching dry weights and growth rates of respectively 0.36, 0.30 g L−1 and 0.85, 0.67 day−1 for the PBR and open pond (Castro-Varela et al. 2021). Chaumont (1993) mentioned a productivity of 0.29 to 0.36 g L−1 day−1 attained for up to 2 months at steady state obtained at a cell concentration of 6 g L−1 (Gudin and Chaumont 1991; Chaumont 1993). One potential explanation for their high productivity and DW obtained could be the tight temperature control by floating or immersing the reactor in a waterbath. A second potential reason is the serpentine configuration of their reactor, avoiding shading of tubes that occurs in horizontal tubular reactors with multiple tubes stacked above each other. However, this type of system would be too costly for most applications (Tredici 2003). Compared with the above-mentioned values from the literature, excluding the short term Rebolloso Fuentes et al. (1999) study and the Gudin and Chaumon (1991, 1993) studies, we observed similar growth rates and productivities. However, compared to several other commercially cultivated algae we obtained lower growth rates and productivities (de Vree et al. 2015; Borowitzka and Vonshak 2017; Benedetti et al. 2018).
If we compare our data to the standard production method for Porphyridium sp., sleeves, utilized by the Arad group, a similar growth rate (0.117 vs 0.115 day−1) was obtained. However, our maximum DW reached was lower (1.94 vs 2.8 g L−1) (Cohen et al. 1989; Arad and Cohen 1991; Cohen et al. 1991). Furthermore, the doubling time, between 1 and 5 days, reported by the Arad group (Arad et al. 1985) was generally lower than the ones reported in this study (Table 1).
In terms of productivity, they obtained respectively for batch and continuous culture in 100 L 0.102 and 0.177 g L−1 day−1, which is a similar result compared to our batch cultivation (0.084 ± 0.044 g L−1 day−1), however, their continuous cultivation was twice the productivity we obtained in our batch cultivation. An effect they also noticed with their higher productivity in continuous versus batch cultivation (Cohen et al. 1989; Arad and Cohen 1991; Cohen et al. 1991). Fuentes-Grünewald et al. (2015) also observed higher growth rates in semi-continuous cultivation (0.047 g L−1 day−1) compared to batch cultivation (0.027 g L−1 day−1) (Fuentes-Grünewald et al. 2015).
Future large-scale cultivation experiments of P. purpureum in tubular photobioreactors will be needed to fully understand its potential and to increase the growth rates. Especially (semi-) continuous cultivation of P. purpureum should be investigated as the (semi-) continuous cultivation has been shown to more productive.
Two important parameters for algal growth are the PAR received and the temperature during cultivation. During our experiments we were however unable to show a clear correlation between the average PAR received, the temperature and growth rate or doubling time (Fig. S2). To compare the growth periods and considering the different total PAR received between the growth periods, we normalized the growth for PAR received by the yield per light (PL). Periods with low average PAR values attained often yields comparable or higher than periods with higher average PAR values (Table 1). During some periods artificial light was provided but a cautionary note is that the artificial light used here was of subpar quality and placed central underneath the reactors (less light reached the higher located tubes of the reactor compared to the lower tubes). With modern LED lighting a more optimized model can be determined regarding extra lighting. However, using artificial light will also increase the cost of production and one of the limiting factors of the microalgae commercialization is the production cost (Moomaw et al. 2017; Latsos et al. 2022).
During our cultivation experiments the average temperatures in the greenhouse stayed relatively stable between 15 and 22 °C during large parts of our cultivation period (Fig. 3, Tables 1 and S2) which is in line with the growth temperatures of mesophilic algae such as Porphyridium species (Gaignard et al. 2019). Multiple laboratory studies have already shown that P. purpureum thrives best at temperatures between 20 and 25 °C (Gaignard et al. 2019; Li et al. 2019a), but can still grow at lower temperatures (Durmaz et al. 2007; Guihéneuf and Stengel 2015). During our pilot-scale test even at 10 °C growth was still noticeable, potentially offering opportunities to cultivate P. purpureum at colder periods. While lower temperatures were well tolerated by the algae, higher temperatures, especially above 35 °C, were however detrimental for its growth (Fig. 3, Tables 1 and S2). Throughout our 2-year cultivation period two cultivations attempted during the summer periods in 2018 and 2019 (23 August-2 September 2018 and 24–31 August 2019) failed due to rapid culture crashes (Fig. 2, red arrows). This was due to max temperatures up to 35–40 °C and with multiple days reaching average temperatures between 32 and 38 °C in the greenhouse. Furthermore, a halt in growth was observed during the 9–29 April batch period after temperatures between 30 and 35.2 °C were reached in the greenhouse. Previously, temperatures from and above 30 °C have been shown to have a negative influence on P. purpureum (Golueke and Oswald 1962). However, some authors reported growth up to 35 °C without problems (Dermoun et al. 1992; Chang et al. 2017). As we measured the ambient temperature in the greenhouse rather than the culture temperature itself, future studies addressing the culture temperature itself are advised since the temperature inside the culture, especially for larger culture volumes, can deviate from the ambient temperature (Tredici and Materassi 1992; González-Camejo et al. 2019).
Under well-controlled laboratory conditions one can keep several parameters constant and change one specific parameter to study its influence on the growth of the algae. However, even at laboratory-scale sparse information is available. Often studies are restricted to one or two factors and almost none describe the interactive effects of multiple parameters for the growth of Porphyridium spp. (Guihéneuf and Stengel 2015). When cultivating in pilot-scale, especially outdoors (in a greenhouse), it is however impossible to tightly regulate the conditions influencing the growth. Since several parameters (e.g., PAR, pH, temperature, day/night cycle, CO2 exchange, flow and mixing) can influence each other and the algal growth, it is more difficult to find a correlation between them. Furthermore, since the composition of the algae depends on the environmental conditions more larger scale tests are needed (Moomaw et al. 2017).
During our pilot-scale tests we used a N/P ratio of 5/1 in our medium, although other ratios might be more interesting to steer the growth or production of specific components (Razaghi et al. 2014). Between 14.5 and 36.6 mg N was consumed per g biomass produced during our pilot-scale tests. If one uses the maximum productivity of 0.195 g L−1 day−1 a total of 2.8–7.1 mg day−1 L−1 N would be needed to sustain the cultivation. For P, 3.2–6.19 mg P consumed per g biomass, a total of 0.6–1.2 mg day−1 L−1 P would be needed. Four large scale-studies only mentioned the medium used but gave no specifics of the N and P consumption (Chaumont 1993; Rebolloso Fuentes et al. 1999; Fuentes-Grünewald et al. 2015; Yin et al. 2022) and the fifth study only looked at the nitrogen consumption at laboratory scale (Castro-Varela et al. 2021) making it impossible to compare our results. In our study we used regular agriculture fertilizers, nitric acid (4 mM) and phosphoric acid (2.5 mM) as a N and P source. To make algae cultivation more sustainable and economical viable one could use wastewaters or liquid sidestreams rich in N and P (Guldhe et al. 2017; Thoré et al. 2021a) or use urea (14.8 mM) as a nitrogen source (Castro-Varela et al. 2021). At the moment however, only limited studies are available on the use of wastewater to cultivate Poprhyridium species (Sandefur et al. 2016; Arashiro et al. 2020; Erol et al. 2020; Zhang et al. 2022) and success was not always guaranteed (Wang et al. 2016). A different approach could be the use of seawater (Borovkov et al. 2022), however nutrients still need to be added and often artificial seawater is used (Gaignard et al. 2019). Nonetheless, these nutrients can partly be substituted by using wastewater (Golueke and Oswald 1962).
Biofilm formation
One major issue with cultivating microalgae is the formation of biofilm. Especially during longer cultivation periods, it is impossible to fully prevent biofouling. Furthermore, the production of extracellular polysaccharides (EPS) can increase the risk of biofilm formation (Zeriouh et al. 2017). The formation of biofilm can become problematic for long-term cultivation of P. purpureum in large-scale photobioreactors. Currently, commercial production often takes place in plastic bags with a 60-to-80-L volume (Fuentes-Grünewald et al. 2015). These bags have higher yields, provide better control of the culture conditions and reduce contamination compared to open pond systems (Cohen et al. 1991; Gaignard et al. 2019; Bayu et al. 2022). The bags or sleeves are most often single use and have a short life span, although they can be used for several months (Cohen et al. 1989; Arad and Cohen 1991), and once the biofilm formation is too severely, they are trashed. However, for large-scale production of biomass the use of photobioreactors with larger working volumes can be of interest. Yet, of the five large-scale studies found in the literature, three studies describe short cultivation periods between 3 and 14 days while the two studies describing longer cultivation periods mentioned nothing specific regarding biofilm formation in the photobioreactors (Gudin and Chaumont 1991; Chaumont 1993; Rebolloso Fuentes et al. 1999; Fuentes-Grünewald et al. 2015; Castro-Varela et al. 2021; Yin et al. 2022). In our study we successfully cultivated P. purpureum up to a total of 136 days without contamination issues. In a second long-term cultivation attempt a successful cultivation period of 76 consecutive days in one reactor (1500 L) was attained. However, when aiming for longer cultivation periods the formation of biofilm was becoming more prevalent and cleaning of the reactors was required to prevent culture crashes or contaminated cultures as the biofilm formation also stimulated the growth of contaminating organisms (Fig. 4). We observed that a successful approach to reduce or prevent the formation of biofilm was to cultivate and harvest P. purpureum at lower cell densities (between 0.7 and 1.0 g L−1). Preventing the cells to reach a stationary phase prior to harvesting can be vital to prevent biofouling as the polysaccharide capsule surrounding the cells is the thickest at stationary phase (Arad and Richmond 2003).
CO2 utilization efficiency at pilot-scale
To evaluate the CO2 utilization efficiency, the total CO2 injected in the reactors during 6 batches was monitored. Efficiencies between 5.1 and 30.9% were found, using the assumption of 43% carbon content in the cells, which is in line with previously reported studies (Tang et al. 2011; Gabriel Acien Fernandez et al. 2012; Schoeters et al. 2022). Two limitations in our study are that we used an assumption of the carbon content and that we did not measure the outgoing CO2 and only measured the ingoing CO2 and calculated our efficiencies based on the produced biomass. For a more correct interpretation the outgoing CO2 should have been measured as well.
Key factors in improving the CO2 efficiency are the reactor design, parameters such as pH, temperature and how the CO2 is distributed into the reactor (e.g., turbulence) (Posten 2009; Acien Fernandez et al. 2012; Morales et al. 2018; Daneshvar et al. 2022). In our first test (9–15 October 2018) CO2 utilizations efficiencies of 5.1 and 30.9% at respectively a pH setpoint of 8.5 and 8.0 were obtained in R3 and R4. While the solubility of CO2 increases with an increasing pH, the utilization efficiencies observed here were the opposite, potentially due to a difference in the physiological state and photosynthetic activity of the algae (Davison 1991; Hancke et al. 2008; Masojídek et al. 2023). Both reactors were constructed the same and aside from the pH, operated similar. Two tests in the same reactor, R3, with the same pH setpoint, 7.5, did result in very similar CO2 utilization efficiencies of 5.8 and 7.6%. While a test in R4 with the same pH setpoint (7.5) lead to a 10.4% efficiency. As both light and temperature can have an influence on the physiological state and photosynthetic activity (Davison 1991; Hancke et al. 2008; Masojídek et al. 2023), small differences in total PAR received and temperature could have influenced the CO2 utilization between the two reactors as these tests were performed at different times. Furthermore, including photosynthesis measuring techniques such as O2 production and in situ Chl fluorescence in future experiments might prove useful in tracking the growth in our system (Masojídek et al. 2023).
Since CO2 utilization depends on so many factors such as growth rate, growth phase, algal species cultivated, temperature, pH, turbulence and culture medium used (Posten 2009; Gabriel Acien Fernandez et al. 2012; Klinthong et al. 2015; Morales et al. 2018), an interesting approach could potentially be to re-capture the CO2 leaving the photobioreactor for re-use. As such a more closed approach can be utilized providing a better usage of the CO2.
Conclusion
In this study we report the successful batch cultivation over a 2 -year period of the red alga P. purpureum in a tubular photobioreactor located in a greenhouse. Cultivation was performed up to a total volume of 1500 L. A maximum dry weight of 1.94 g L−1 was obtained while a maximum growth rate and a maximum productivity of 0.233 day−1 and 0.195 g L−1 day−1, respectively, were achieved. During our cultivation temperatures above 34–35 °C were found to be critical, leading to inhibition of the growth or even causing a rapid crash of P. purpureum cultivation as observed during the failed cultivation attempts in the summers of 2018 and 2019. Temperatures as low as 10—15 °C however still supported growth. Since most commercially cultivated microalgae have optimal growth temperatures between 20 and 35 °C, hampering cultivation in regions with lower average temperatures, the cultivation of P. purpureum could thus provide an alternative, albeit at lower productivities. During our experiments we only looked at the batch growth of P. purpureum as such and did not investigate a more continuous cultivation in a horizontal tubular reactor. As previous studies have shown that a (semi-) continuous approach can lead to higher productivities, future studies should investigate the (semi-) continuous versus the batch method approach for P. purpureum cultivation in tubular photobioreactors. Furthermore, nitric acid was used as a nitrogen source while for commercial large-scale cultivation more economical feasible alternatives, such as urea, should be investigated. More studies should address the above to further elucidate the potential of large-scale and long-term cultivation of P. purpureum in horizontal tubular reactors in outdoor conditions.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Acien Fernandez FG, González-López CV, Fernández Sevilla JM, Molina Grima E (2012) Conversion of CO2 into biomass by microalgae: How realistic a contribution may it be to significant CO2 removal? Appl Microbiol Biotechnol 96:577–586
Ahlgren G, Gustafsson I-B, Boberg M (1992) Fatty acid content and chemical composition of freshwater microalgae. J Phycol 28:37–50
Arad S, Cohen E (1991) Outdoor cultivation of microalgae in a closed system for the production of valuable biochemicals. In: Kamely D, Chakrabarty AM, Kornguth SE (eds) Biotechnology: Bridging Research and Applications. Springer, Dordrecht, pp 301–316
Arad S (Malis), Adda M, Cohen E (1985) The potential of production of sulfated polysaccharides from Porphyridium. Plant Soil 89:117–127
Arad S, Richmond A (2003) Industrial production of microalgal cell-mass and secondary products - species of high potential: Porphyridium sp. In: Richmond A (ed) Handbook of Microalgal Culture. Blackwell Science, Oxford, pp 289–297
Arashiro LT, Boto-Ordóñez M, van Hulle SWH, Ferrer I, Garfi M, Pousseau PL (2020) Natural pigments from microalgae grown in industrial wastewater. Bioresour Technol 303:122894
Araújo R, Vázquez Calderón F, Sánchez López J, Azevedo IC, Bruhn A, Fluch S, Garcia Tasende M, Ghaderiardakani F, Ilmjärv T, Laurans M, Mac Monagail M, Mangini S, Peteiro C, Rebours C, Stefansson T, Ullmann J (2021) Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front Mar Sci 7:626389
Bayu A, Noerdjito DR, Rahmawati SI, Putra MY, Karnjanakom S (2022) Biological and technical aspects on valorization of red microalgae genera Porphyridium. Biomass Conv Biorefin. https://doi.org/10.1007/s13399-021-02167-5
Benedetti M, Vecchi V, Barera S, Dall’Osto L, (2018) Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact 17:173
Bhalamurugan GL, Valerie O, Mark L (2018) Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ Eng Res 23:229–241
Borovkov AB, Gudvilovich IN, Novikova TM, Klimova EV (2022) Production characteristics of Porphyridium purpureum (Bory) Drew et Ross semi-continuous culture at low irradiance. Mar Biol J 7:3–13
Borowitzka MA, Vonshak A (2017) Scaling up microalgal cultures to commercial scale. Eur J Phycol 52:407–418
Brody M, Emerson R (1959) The effect of wavelength and intensity of light on the proportion of pigments in Porphyridium cruentum. Am J Bot 46:433–440
Castro-Varela P, Sáez K, Gómez PI (2021) Effect of urea on growth and biochemical composition of Porphyridium purpureum (Rhodophyta) and scaling-up under non-optimal outdoor conditions. Phycologia 60:572–581
Chang J, Le K, Song X, Jiao K, Zeng X, Ling X, Shi T, Tang X, Sun Y, Lin L (2017) Scale-up cultivation enhanced arachidonic acid accumulation by red microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 40:1763–1773
Chaumont D (1993) Biotechnology of algal biomass production: a review of systems for outdoor mass culture. J Appl Phycol 5:593–604
Chaumont D, Thepenier C, Gudin C, Junjas C (1988) Scaling up a tubular photoreactor for continuous culture of Porphyridium cruentum from laboratory to pilot plant (1981–1987). In: Stadler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiaen D (eds) Algal Biotechnology. Elsevier, London, pp 199–208
Cohen E, Arad S (Malis) (1989) A closed system for outdoor cultivation of Porphyridium. Biomass 18:59–67
Cohen E, Koren A, (Malis) Arad S (1991) A closed system for outdoor cultivation of microalgae. Biomass Bioenerg 1:83–88
Daneshvar E, Wicker RJ, Show P-L, Bhatnagar A (2022) Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization – A review. Chem Eng J 427:130884
Davison IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27:2–8
de Vree JH, Bosma R, Janssen M, Barbosa MJ, Wijffels RH (2015) Comparison of four outdoor pilot-scale photobioreactors. Biotechnol Biofuels 8:215
Dermoun D, Chaumont D, Thebault J-M, Dauta A (1992) Modelling of growth of Porphyridium cruentum in connection with two interdependent factors: Light and temperature. Bioresour Technol 42:113–117
Dolganyuk V, Belova D, Babich O, Prosekov A, Ivanova S, Katserov D, Patyukov N, Sukhikh S (2020) Microalgae: A promising source of valuable bioproducts. Biomolecules 10:1513
Durmaz Y, Monteiro M, Bandarra N, Gökpinar S, Isik O (2007) The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J Appl Phycol 19:223–227
Erol HBU, Menegazzo ML, Sandefur H, Gottberg E, Vaden J, Asgharpour M, Hestekin CN, Hestekin JA (2020) Porphyridium cruentum grown in ultra-filtered swine wastewater and its effects on microalgae growth productivity and fatty acid composition. Energies 13:3194
Fernández FGA, Camacho FG, Pérez JAS, Sevilla MF, Grima EM (1998) Modeling of biomass productivity in tubular photobioreactors for microalgal cultures: Effects of dilution rate, tube diameter, and solar irradiance. Biotechnol Bioeng 58:605–616
Fuentes-Grünewald C, Bayliss C, Zanain M, Pooley C, Scolamacchia M, Aal S (2015) Evaluation of batch and semi-continuous culture of Porphyridium purpureum in a photobioreactor in high latitudes using Fourier Transform Infrared spectroscopy for monitoring biomass composition and metabolites production. Bioresour Technol 189:357–363
Gaignard C, Gargouch N, Dubessay P, Delattre C, Pierre G, Laroche C, Fendri I, Abdelkafi S, Michaud P (2019) New horizons in culture and valorization of red microalgae. Biotechnol Adv 37:193–222
Goldman JC, Porcella DB, Middlebrooks JE, Toerien DF (1971) The effect of carbon on algal growth-its relationship to eutrophication. Utah Water Research laboratory, Utah State University. Paper 462. https://digitalcommons.usu.edu/water_rep
Golueke CG, Oswald WJ (1962) The mass culture of Porphyridium cruentum. Appl Microbiol 10:102–107
González-Camejo J, Aparicio S, Ruano MV, Borrás L, Barat R, Ferrer J (2019) Effect of ambient temperature variations on an indigenous microalgae-nitrifying bacteria culture dominated by Chlorella. Bioresour Technol 290:121788
Gudin C, Chaumont D (1991) Cell fragility - The key problem of microalgae mass production in closed photobioreactors. Bioresour Technol 38:145–151
Guihéneuf F, Stengel DB (2015) Towards the biorefinery concept: Interaction of light, temperature and nitrogen for optimizing the co-production of high-value compounds in Porphyridium purpureum. Algal Res 10:152–163
Guiry MD (2012) How many species of algae are there? J Phycol 48:1057–1063
Guldhe A, Kumari S, Ramanna L, Ramsundar P, Singh P, Rawat I, Bux F (2017) Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. J Environ Manage 203:299–315
Hancke K, Hancke TB, Olsen LM, Johnses G, Glud RN (2008) Temperature effects on microalgal photosynthesis-light responses measured by O2 production, pulse-amplitude-modulated fluorescence, and 14C assimilation. J Phycol 44:501–514
Ho SH, Chen YD, Chang CY, Lai YY, Chen CY, Kondo A, Ren NQ, Chang JS (2017) Feasibility of CO2 mitigation and carbohydrate production by microalga Scenedesmus obliquus CNW-N used for bioethanol fermentation under outdoor conditions: effects of seasonal changes. Biotechnol Biofuels 10:27
Islam Z, Khatoon H, Minhaz TM, Rahman MR, Hasan S, Mahmud Y, Hossain MS, Sarker J (2021) Data on growth, productivity, pigments and proximate composition of indigenous marine microalgae isolated from Cox’s Bazar Coast. Data Brief 35:106860
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:36
Klinthong W, Yang YH, Huang CH, Tan CS (2015) A review: Microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual Res 15:712–742
Latsos C, Wassenaar E, Moerdijk T, Coleman B, Robbens J, van Roy S, Bastiaens L, van Houcke J, Timmermans KR (2022) Effect of pH on Rhodomonas salina growth, biochemical composition, and taste, produced in semi-large scale under sunlight conditions. J Appl Phycol 24:1215–1226
Lee ETY, Bazin MJ (1991) Environmental factors influencing photosynthetic efficiency of the micro red alga Porphyridium cruentum (Agardh) Nägeli in light-limited cultures. New Phytol 118:513–519
Lee Y-K, Shen H (2003) Basic culturing techniques. In: Richmond A (ed) Handbook of Microalgal Culture. Blackwell Science, Oxford, pp 40–56
Li S, Ji L, Shi Q, Wu H, Fan J (2019a) Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour Technol 292:122048
Li T, Xu J, Wu H, Jiang P, Chen Z, Xiang W (2019b) Growth and biochemical composition of Porphyridium purpureum SCS-02 under different nitrogen concentrations. Mar Drugs 17:124
Li Z, Bock R (2018) Replication of bacterial plasmids in the nucleus of the red alga Porphyridium purpureum. Nat Commun 9:3451
Masojídek J, Štěrbová K, Serrano CG, da Silva JC, Grivalský T, Figueroa FL, Fernández FGA (2023) Photosynthetic performance of Chlamydopodium (Chlorophyta) cultures grown in outdoor bioreactors. Appl Microbiol Biot 107:2249–2262
Merlo S, Gabarrell Durany X, Pedroso Tonon A, Rossi S (2021) Marine microalgae contribution to sustainable development. Water 13:1373
Molina Grima E, García Camacho F, Sánchez Pérez JA, Acién Fernández FG, Fernández Sevilla JM (1997) Evaluation of photosynthetic efficiency in microalgal cultures using averaged irradiance. Enzyme Microb Technol 21:375–381
Moomaw W, Berzin I, Tzachor A (2017) Cutting out the middle fish: Marine microalgae as the next sustainable omega-3 fatty acids and protein source. Indust Biotechnol 13:234–243
Moraes L, da Rosa GM, Cardias BB, dos Santos LO, Costa JAV (2016) Microalgal biotechnology for greenhouse gas control: Carbon dioxide fixation by Spirulina sp. at different diffusers. Ecol Eng 91:426–431
Morales M, Sánchez L, Revah S (2018) The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol Lett 365(3):fnx262
Pérez-López P, de Vree JH, Feijoo G, Bosma R, Barbosa MJ, Moreira MT, Wijffels RH, van Boxtel AJB, Kleinegris DMM (2017) Comparative life cycle assessment of real pilot reactors for microalgae cultivation in different seasons. Appl Energy 205:1151–1164
Perrineau MM, Price DC, Mohr G, Bhattacharya D (2015) Recent mobility of plastid encoded group II introns and twintrons in five strains of the unicellular red alga Porphyridium. PeerJ 3:e1017
Posten C (2009) Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci 9:165–177
Quinn J, de Winter L, Bradley T (2011) Microalgae bulk growth model with application to industrial scale systems. Bioresour Technol 102:5083–5092
Razaghi A, Godhe A, Albers E (2014) Effects of nitrogen on growth and carbohydrate formation in Porphyridium cruentum. Cent Eur J Biol 9:156–162
Rebolloso Fuentes MM, Garcia Sánchez JL, Fernández Sevilla JM, Acién Fernandez FG, Sánchez Pérez JA, Molina Grima E (1999) Outdoor continuous culture of Porphyridium cruentum in a tubular photobioreactor: quantitative analysis of the daily cyclic variation of culture parameters. J Biotechnol 70:271–288
Reitan KI, Øie G, Jørgensen H, Wang X (2021) Chemical composition of selected marine microalgae, with emphasis on lipid and carbohydrate production for potential use as feed resources. J Appl Phycol 33:3831–3842
Richmond A (2000) Microalgal biotechnology at the turn of the millennium: A personal view. J Appl Phycol 12:441–451
Sánchez Mirón A, Cerón García MC, Contreras Gómez A, Garcia Camacho F, Molina Grima E, Chisti Y (2003) Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem Eng J 16:287–297
Sandefur HN, Asgharpour M, Mariott J, Gottberg E, Vaden J, Matlock M, Hestekin J (2016) Recovery of nutrients from swine wastewater using ultrafiltration: Applications for microalgae cultivation in photobioreactors. Ecol Eng 94:75–81
Sato N, Moriyama T, Mori N, Toyoshima M (2017) Lipid metabolism and potentials of biofuel and high added-value oil production in red algae. World J Microbiol Biotechnol 33:74
Schoeters F, Spit J, Azizah RN, van Miert S (2022) Pilot-scale cultivation of the snow alga Chloromonas typhlos in a photobioreactor. Front Bioeng Biotechnol 10:896261
Singh S, Arad S, (Malis), Richmond A, (2000) Extracellular polysaccharide production in outdoor mass cultures of Porphyridium sp. in flat plate glass reactors. J Appl Phycol 12:269–275
Singh UB, Ahluwalia AS (2013) Microalgae: a promising tool for carbon sequestration. Mitig Adapt Strateg Glob Change 18:73–95
Tang D, Han W, Li P, Miao X, Zhoing J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Thoré ESJ, Schoeters F, de Cuyper A, Vleugels R, Noyens I, Bleyen P, Van Miert S (2021a) Waste is the new wealth – Recovering resources from poultry wastewater for multifunctional microalgae feedstock. Front Environ Sci 9:679917
Thoré ESJ, Schoeters F, Spit J, Van Miert S (2021b) Real-time monitoring of microalgal biomass in pilot-scale photobioreactors using nephelometry. Processes 9:1530
Tredici MR (2010) Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 1:143–162
Tredici MR (2003) Mass production of microalgae: Photobioreactors. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. Blackwell Publishing, Oxford, pp 40–56
Tredici MR, Materassi R (1992) From open ponds to vertical alveolar panels: the Italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. J Appl Phycol 4:221–231
Vecchi V, Barera S, Bassi R, Dall’Osto L (2020) Potential and challenges of improving photosynthesis in algae. Plants 9:67
Wang M, Yang Y, Chen Z, Chen Y, Wen Y, Chen B (2016) Removal of nutrients from undiluted anaerobically treated piggery wastewater by improved microalgae. Bioresour Technol 222:130–138
Yin HC, Sui JK, Han TL, Liu TZ, Wang H (2022) Integration bioprocess of B-phycoerythrin and exopolysaccharides production from photosynthetic microalga Porphyridium cruentum. Front Mar Sci 8:836370
You T, Barnett SM (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19:251–258
Zeriouh O, Reinoso-Moreno JV, López-Rosales L, Cerón-García MDC, Sánchez-Mirón A, García-Camacho F, Molina-Grima E (2017) Biofouling in photobioreactors for marine microalgae. Crit Rev Biotechnol 37:1006–1023
Zhang AH, Feng B, Zhang H, Jiang J, Zhang D, Du Y, Chng Z, Huang J (2022) Efficient cultivation of Porphyridium purpureum integrated with swine wastewater treatment to produce phycoerythrin and polysaccharide. J Appl Phycol 34:2315–2326
Acknowledgements
The authors would like to thank S. Goossens for helping with sampling for acquisition of data. R. N. Azizah for help with data analysis. A. Wuyts for helping with cultivation and upscaling of P. purpureum. E. Van Cantfort for helping with the figures.
Funding
This research was funded by NORTH-WEST EUROPE INTERREG, grant number NWE 639 as part of the IDEA project (Implementation and development of economic viable algae-based value chains in North-West Europe).
Author information
Authors and Affiliations
Contributions
FS: Formal analysis and interpretation, Acquisition of data; Writing—original draft, writing – review & editing; JS: Conceptualization, Formal analysis and interpretation, Acquisition of data, Writing—review & editing; ES: Formal analysis and interpretation, Writing—original draft, writing – review & editing; AC: Formal analysis and interpretation, Writing—original draft, writing – review & editing; RV: Acquisition of data, Writing—review & editing; IN: Acquisition of data, Writing—original draft, Writing—review & editing; SM: Funding acquisition, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schoeters, F., Spit, J., Swinnen, E. et al. Pilot-scale cultivation of the red alga Porphyridium purpureum over a two-year period in a greenhouse. J Appl Phycol 35, 2095–2109 (2023). https://doi.org/10.1007/s10811-023-03045-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03045-5