Abstract
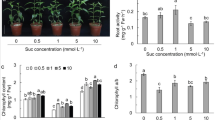
The effects of different types and concentrations of sugars on root growth and xanthone production in root culture of Gentiana dinarica were investigated. The results showed that sucrose, glucose and fructose all supported root growth, and sucrose was superior in terms of growth index, dry mass and fresh/dry mass ratio then fructose or glucose at the same concentrations. However, considering equimolar concentration of sugars, their contribution to the root growth was similar. The HPLC analysis of roots indicated the presence of xanthone compounds, and the contents of norswertianin-1-O-primeveroside (1), norswertianin-1-O-glucoside (2), gentioside (3) and norswertianin (4) were evaluated. In all samples, norswertianin-1-O-primeveroside (1) was present in highest concentration, followed by norswertianin-1-O-glucoside (2), whereas gentioside (3) and norswertianin (4) were present in lower amounts. The production of xanthones was affected by both type and concentration of sugar. In general, roots growing in media supplemented with sucrose contained higher levels of xanthones. The amounts of xanthone primeveroses (1) and (3) increased with the increase of concentrations of all types of sugars, whereas higher sugar concentrations resulted in reduction of the contents of norswertianin-1-O-glucoside (2) and aglycone norswertianin (4). The roots were also evaluated regarding the content of total phenolics and higher accumulation of total phenolic compounds was observed in roots grown in fructose-containing medium. Antioxidant activity was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, and high correlation between total phenolic content and antiradical activity was observed (r = −0.83).





Similar content being viewed by others
References
Beerhues L, Berger U (1995) Differential accumulation of xanthones in methyl-jasmonate- and yeast extract-treated cell cultures of Centaurium erythraea and Centaurium littorale. Planta 197:608–612
Bennett G, Lee H-H (1988) The biosynthesis of mangostin: the origin of the xanthone skeleton. J Chem Soc Chem Commun 619–620
Brand-Williams W, Cuvelier ME, Berset C (1995) Use a free radical method to evaluate antioxidative activity. LWT Food Sci Technol 28:25–30
Butcher DN, Street HE (1964) Excised root culture. Bot Rev 30:513–586
Chueh F-S, Chen C-C, Sagare A, Tsay H-S (2001) Quantitative determination of secoiridoid glucosides in in vitro propagated plants of Gentiana davidii var. formosana by high performance liquid chromatography. Planta Med 67:70–73
Corrêa L, Paim DC, Schwambach J, Fett-Neto AG (2005) Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globules Labill. Plant Growth Regul 45:63–73
Dević M, Momčilović I, Krstić D, Maksimović V, Konjević R (2006) In vitro multiplication of willow gentian (Gentiana asclepiadea L.) and the production of gentiopicrine and mangiferin. Phyton 46:45–54
Drew RA, Considine JA, McComb JA (1993) Effect of fructose on growth of papaw shoot explants in vitro. Aust J Bot 41:739–748
Gruselle R, Nicaise C, Boxus P (1995) Regulation of in vitro shoot multiplication in Persian walnut by different carbon sources and by ammonium phosphate. Bull Rech Agron Gembloux 30:47–53
Hosokawa K, Oikawa Y, Yamamura S (1998) Mass propagation of ornamental gentian in liquid medium. Plant Cell Rep 17:747–751
Hostettmann-Kaldas M, Hostettmann K, Sticher O (1981) Xanthones, flavones and secoiridoids of American Gentiana species. Phytochemistry 20:443–446
Janković T, Krstić D, Šavikin-Fodulović K, Menković N, Grubišić D (2000) Xanthone compounds of Centaurium erythraea grown in nature and cultured in vitro. Pharm Pharmacol Lett 10:23–25
Janković T, Vinterhalter B, Krstić-Milošević D, Nikolić R, Vinterhalter D, Milosavljević S (2011) Xanthone compounds in shoot cultures of Gentianella bulgarica. Acta Physiol Plant 33:1515–1520
Jensen SR, Schripsema J (2002) Chemotaxonomy and pharmacology of Gentianaceae. In: Albert VA (ed) Struwe L. Systematics and Natural History, Cambridge University press, Gentianaceae, pp 573–631
Kim JH, Yun JH, Hwang YS, Byun SY, Kim DI (1995) Production of taxol and related taxanes in Taxus brevifolia cell cultures: effect of sugar. Biotechnol Lett 17:101–106
Kim SI, Choi HK, Kim JH, Lee HS, Hong SS (2001) Effect of osmotic pressure on paclitaxel production in suspension cell cultures of Taxus chinensis. Enzyme Microb Tech 28:202–209
Krstić D, Janković T, Šavikin-Fodulović K, Menković N, Grubišić D (2003) Secoiridoids and xanthones in the shoots and roots of Centaurium pulchellum cultured in vitro. In Vitro Cell Dev Biol Plant 39:203–207
Krstić D, Janković T, Aljančić I, Šavikin-Fodulović K, Menković N, Milosavljević S (2004) Phytochemical investigation of Gentiana dinarica. Biochem Syst Ecol 32:937–941
Legha MR, Prasad KV, Singh SK, Kaur C, Arora A, Kumar S (2012) Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell Dev Biol Plant 48:99–106
Liu C-Z, Cheng X-Y (2008) Enhancement of phenylethanoid glycosides biosynthesis in cell cultures of Cistanche deserticola by osmotic stress. Plant Cell Rep 27:357–362
Menković N, Šavikin-Fodulović K, Vinterhalter B, Vinterhalter D, Grubišić D (1998) Secoiridoid content of naturally grown and in vitro cultured Gentiana punctata. Pharm Pharmacol Lett 8:110–111
Menković N, Šavikin-Fodulović K, Momčilović I, Grubišić D (2000) Quantitative determination of secoiridoid and γ-pyrone compounds in Gentiana lutea cultured in vitro. Planta Med 66:96–98
Mikula A, Rybczynski J (2001) Somatic embyogenesis of Gentiana genus. The effect of the preculture treatment and primary explant origin on somatic embryogenesis of Gentiana cruciata (l.), G. pannonica (Scop.), and G. tibetica (King). Acta Physiol Plant 23:15–25
Mišić D, Maksimović V, Todorović S, Grubišić D, Konjević R (2005) Influence of carbohydrate source on Nepeta rtanjensis growth, morphogenesis, and nepetalactone production in vitro. Isr J Plant Sci 53:103–108
Momčilović I, Grubišić D, Nešković N (1997) Micropropagation of four Gentiana species (G. lutea, G. cruciata, G. purpurea and G. acaulis). Plant Cell Tiss Org Cult 49:141–144
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pawlowska B, Bach A (2003) In vitro propagation of protected species Gentiana pneumonanthe L. for ornamental horticultural use. Folia Horticulturae 15:113–122
Pinto MMM, Sousa ME, Nascimento MSJ (2005) Xanthone derivatives: new insights in biological activities. Curr Med Chem 12:2517–2538
Pontus S, Michael AP, Chaim I (2006) Use of Gentiana lutea extracts as an antimicrobial agent. European Patent EP1663271
Rice-Evans C, Miller N, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Romano A, Noronha C, Martins-Loução MA (1995) Role of carbohydrates in micropropagation of cork oak. Plant Cell Tiss Org Cult 40:159–167
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–153
Struwe L, Kadereit JW, Klackenberg J, Nilsson S, Thiv M, von Hagen B, Albert VA (2002) Systematics, character evolution and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: Albert VA (ed) Struwe L. Systematics and Natural History, Cambridge University Press, Gentianaceae, pp 21–309
Vieira LMM, Kijjoa A (2005) Naturally-occurring xanthones: recent developments. Curr Med Chem 12:2413–2446
Vinterhalter B, Vinterhalter D (1998) In vitro propagation of spotted gentian Gentiana punctata L. Arch Biol Sci 50(3):177–182
Vinterhalter B, Janković T, Šavikin K, Nikolić R, Vinterhalter D (2008) Propagation and xanthone content of Gentianella austriaca shoot cultures. Plant Cell Tiss Org Cult 94:329–335
Vinterhalter B, Krstić-Milošević D, Janković T, Milojević J, Vinterhalter D (2012) In vitro propagation of Gentiana dinarica Beck. Cent Eur J Biol 7:690–697
Vinterhalter B, Krstić-Milošević D, Janković T, Zdravković-Korać S, Vinterhalter D (2013) Quantitative determination of secoiridoid and xanthone glycosides of Gentiana dinarica Beck cultured in vitro. Acta Physiol Plant 35:567–574
White PR (1940) Sucrose versus dextrose as carbohydrate source for excised tomato roots. Plant Physiol 15:355–358
Yasodha R, Kamala S, Kumar SP, Kumar PD, Kalaiarasi K (2008) Effect of glucose on in vitro rooting of mature plants of Bambusa nutans. Sci Hortic-Amsterdam 116:113–116
Zwayyed SK, Frazier GC, Dougall DK (1991) Growth and anthocyanin accumulation in carrot cell suspension cultures growing on fructose, glucose, or their mixtures. Biotechnol Prog 7:288–290
Acknowledgments
The authors acknowledge their gratitude to the Ministry of Education, Science and Tecnological Development of Serbia for financial support, project number 173015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krstić-Milošević, D., Janković, T., Vinterhalter, B. et al. Influence of carbohydrate source on xanthone content in root cultures of Gentiana dinarica Beck. Plant Growth Regul 71, 147–155 (2013). https://doi.org/10.1007/s10725-013-9815-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9815-6