Abstract
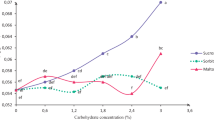
In vitro carotenoid pigment production in callus cultures of Calendula officinalis L. was investigated using two basal media, semi-solid versus liquid media and varied concentrations of sucrose, ammonium, and nitrate nitrogen. Of the two explants that were evaluated, floret explants were best for callus induction using Murashige and Skoog (MS) medium supplemented with 2.0 mg l−1 2,4-dichlorophenoxyacetic acid under complete darkness. Carotenoid pigment induction was significantly augmented when the sucrose concentration was increased. Low sucrose concentrations in the culture medium deferred the onset of pigment induction and reduced the overall levels of carotenoid pigments produced. The highest amount of carotenoid pigments was observed when the callus was grown on the MS medium without ammonium nitrogen. The quantity of carotenoids was slightly elevated in cultures grown on semi-solid medium than those grown in liquid medium. In vitro carotenoid production was optimized by modifying the concentration of ammonium nitrogen to nitrate nitrogen in the culture medium and enhancing the sucrose concentration.




Similar content being viewed by others
References
Aksu Z.; Eren A. T. Carotenoids production by the yeast Rhodotorula mucilaginosa: use of agricultural wastes as a carbon source. Process Biochem 40: 2985–2991; 2005. doi:10.1016/j.procbio.2005.01.011.
Aksu Z.; Eren A. T. Production of carotenoids by the isolated yeast of Rhodotorula glutinis. Biochem Eng J 35: 107–113; 2007. doi:10.1016/j.bej.2007.01.004.
Bartley G. E.; Scolnik P. A. Plant carotenoids: pigments for photoprotection, visual attraction, and human health. The Plant Cell 7: 1027–1038; 1995.
Botella-Pavía P.; Rodríguez-Concepión M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plant 126: 369–381; 2006. doi:10.1111/j.1399-3054.2005.00632.x.
Britton G. Overview of carotenoid biosynthesis. In: Britton G.; Liaaen Jensen S.; Pfander H. (eds) Carotenoid. Birkhauser, Basel, pp 13–147; 1998.
Earle F. R.; Mikolajczak K. L.; Wolff I. A.; Barclay A. S. Search for new industrial oils. X. Seed oils of the Calenduleae. J Amer Oil Chem Soc 41: 345–347; 1964. doi:10.1007/BF02654810.
Fraser P. D.; Bramley P. M. The biosynthesis and nutritional uses of carotenoid. Prog Lipid Res 43: 228–265; 2004. doi:10.1016/j.plipres.2003.10.002.
Gazim Z. C.; Rezende C. M.; Fraga S. R.; Svidzinski T. I. E.; Cortez D. A. G. Antifungal activity of the essential oil from Calendula officinalis L. (Asteraceae) growing in Brazil. Brazilian J Microbiol 39: 61–63; 2008.
George P. S.; Ravishankar G. A. Induction of crocin and crocetins in callus cultures of Gardenia jasminoides Ellis. Food Biotechnol 9: 29–38; 1995. doi:10.1080/08905439509549883.
Giuliano G.; Tavazza R.; Diretto G.; Beyer P.; Taylor M. A. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol 26: 139–145; 2008. doi:10.1016/j.tibtech.2007.12.003.
Gomez K. A.; Gomez A. A. Statistical procedures for agricultural research. 2nd ed. Wiley, New York; 1984.
Grzelak A.; Janiszowska W. Initiation and growth characteristics of suspension cultures of Calendula officinalis cells. Plant Cell Tiss Org Cult 71: 29–40; 2002.
Hussein G.; Sankawa U.; Goto H.; Matsumoto K.; Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod 69: 443–449; 2006.
Jayaraj J.; Punja Z. K. Transgenic carrot plants accumulating ketocarotenoids show tolerance to UV and oxidative stresses. Plant Physiol Biochem 46: 875–883; 2008. doi:10.1016/j.plaphy.2008.05.015.
Jegadeeswari V.; Indurani C.; Kalaiselvi T. Calendula: “the poor man’s saffron”. Science Tech Entrepreneur. Tamil Nadu Agricultural University, Coimbatore; 2007.
Just B. J.; Santos C. A. F.; Fonseca M. E. N.; Boiteux L. S.; Oloizia B. B.; Simon P. W. Carotenoid biosynthesis structural genes in carrot (Daucus carota): isolation, sequence-characterization, single nucleotide polymorphism (SNP) markers and genome mapping. Theor Appl Genet 114: 693–704; 2007. doi:10.1007/s00122-006-0469-x.
Landrum J. T.; Bone R. A. Dietary lutein and zeaxanthin: reducing the risk for macular degeneration. Agro Food Industry Hi-Tech 15: 22–25; 2004.
Li L.; Lu S.; Cosman K. M.; Earle E. D.; Garvin D. F.; O’Neill J. β-Carotene accumulation induced by the cauliflower Or gene is not due to an increased capacity of biosynthesis. Phytochem 67: 1177–1184; 2006. doi:10.1016/j.phytochem.2006.05.013.
Liu B. H.; Lee Y. K. Secondary carotenoids formation by the green alga Chlorococcum sp. J Appl Phycol 12: 301–307; 2000.
Lu S.; Li L. Carotenoid metabolism: biosynthesis, regulation, and beyond. J Integrative Plant Biol 50: 778–785; 2008.
Makunga N. P.; van Staden J.; Cress W. A. The effect of light and 2,4-D on anthocyanin production in Oxalis reclinata callus. Plant Growth Reg 23: 153–158; 1997.
Matkowski A. Plant in vitro culture for the production of antioxidants—a review. Biotech Adv 26: 548–560; 2008. doi:10.1016/j.biotechadv.2008.07.001.
Mizukami H.; Tomita K.; Ohashi H.; Hiraoka N. Anthocyanin production in callus cultures of roselle (Hibiscus sabdariffa L.). Plant Cell Rep 7: 553–556; 1988.
Mok M. C.; Gabelman W. H.; Skoog F. Carotenoid synthesis in tissue cultures of Daucus carota L. J Am Soc Hortic Sci 101: 442–449; 1976.
Mukherjee S. K.; Rathinasabapathi B.; Gupta N. Low sugar and osmotic requirements for shoot regeneration from leaf pieces of Solanum melongena L. Plant Cell Tiss Org Cult 25: 13–16; 1991. doi:10.1007/BF00033906.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497; 1962. doi:10.1111/j.1399-3054.1962.tb08052.x.
Nakagawa K.; Fukui H.; Tabata M. Hormonal regulation of berberine production in cell suspension cultures of Thalictrum minus. Plant Cell Rep 5: 69–71; 1986. doi:10.1007/BF00269722.
Papaioannou E. H.; Liakopoulou-Kyriakides M. Substrate contribution on carotenoid production in Blakeslea trispora cultivations. Food Bioprod Process 88: 305–311; 2010. doi:10.1016/j.fbp.2009.03.001.
Parajó J. C.; Santos V.; Vázquez M. Optimization of carotenoid production by Phaffia rhodozyma cells grown on xylose. Process Biochem 33: 181–187; 1998. doi:10.1016/S0032-9592(97)00045-9.
Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. 2nd ed. Tata McGraw-Hill, New Delhi, pp 83–104; 1996.
Rao S. R.; Ravishankar G. A. Plant cell cultures: chemical factories of secondary metabolites. Biotech Adv 20: 101–153; 2002. doi:10.1016/S0734-9750(02)00007-1.
Sandmann G.; Römer S.; Fraser P. D. Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants—mini review. Metabolic Eng 8: 291–302; 2006. doi:10.1016/j.ymben.2006.01.005.
Shimizu K.; Kikuchi T.; Sugano N.; Nishi A. Carotenoid and steroid syntheses by carrot cells in suspension culture. Physiol Plant 46: 127–132; 2006. doi:10.1111/j.1399-3054.1979.tb06544.x.
Snedecor G. W.; Cochran W. G. Statistical methods. 6th ed. Iowa State University Press, Ames; 1967.
Solovchenko A. E.; Khozin-Goldberg I.; Didi-Cohen S.; Cohen Z.; Merzlyak M. N. Effects of light and nitrogen starvation on the content and composition of carotenoids of the green microalga Parietochloris incisa. Russian J Plant Physiol 55: 455–462; 2008. doi:10.1134/S1021443708040043.
Somashekar D.; Joseph R. Inverse relationship between carotenoid and lipid formation in Rhodotorula gracilis according to the C/N ratio of the growth medium. World J Microbiol Biotech 16: 491–493; 2000. doi:10.1023/A:1008917612616.
Sugano N.; Miya S.; Nishi A. Carotenoid synthesis in a suspension culture of carrot cells. Plant Cell Physiol 12: 525–531; 1971.
Télef N.; Stammitti-Bert L.; Mortain-Bertrand A.; Maucourt M.; Carde J. P.; Rolin D.; Gallusci P. Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp discs. Plant Mol Biol 62: 453–469; 2006. doi:10.1007/s11103-006-9033-y.
Valduga E.; Valério A.; Treichel H.; Júnior A. F.; Luccio M. D. Optimization of the production of total carotenoids by Sporidiobolus salmonicolor (CBS 2636) using response surface technique. Food Bioprocess Technol 2: 415–421; 2009. doi:10.1007/s11947-008-0066-x.
Yamamoto Y.; Kinoshita Y.; Watanabe S.; Yamada Y. Anthocyanin production in suspension cultures of high-producing cells of Euphorbia millii. Agric Biol Chem 53: 417–423; 1989.
Yun J. W.; Kim J. H.; Yoo Y. J. Optimizations of carotenoid biosynthesis by controlling sucrose concentration. Biotech Lett 12: 905–910; 1990. doi:10.1007/BF01022588.
Acknowledgments
MRL acknowledges the grant of Junior Research Fellowship from the Indian Council of Agricultural Research, New Delhi. The author thanks Dr. V. Paul, Division of Plant Physiology, IARI for extending his research facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Finer
Rights and permissions
About this article
Cite this article
Legha, M.R., Prasad, K.V., Singh, S.K. et al. Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell.Dev.Biol.-Plant 48, 99–106 (2012). https://doi.org/10.1007/s11627-011-9402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-011-9402-3