Abstract
Plants contain an extended group of lectins differing from each other in their molecular structures, biochemical properties and carbohydrate-binding specificities. The heterogeneous group of plant lectins can be classified in several families based on the primary structure of the lectin domain. All proteins composed of one or more lectin domains, or having a domain architecture including one or more lectin domains in combination with other protein domains can be defined as lectins. Plant lectins reside in different cell compartments, and depending on their location will encounter a large variety carbohydrate structures, allowing them to be involved in multiple biological functions. Over the years lectins have been studied intensively for their carbohydrate-binding properties and biological activities, which also resulted in diverse applications. The present overview on plant lectins especially focuses on the structural and functional characteristics of plant lectins and their applications for crop improvement, glycobiology and biomedical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
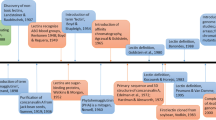
Historical perspective: more than 130 years of plant lectin research
Plant lectins are a heterogeneous group of proteins with a very long history. In 1888 Peter Hermann Stillmark reported the first lectin, when he isolated ricin from the seeds of castor bean (Ricinus communis L.), and described this protein as an extremely toxic compound [1]. Clumping of cells was observed when partially purified seed extracts from castor bean were mixed with red blood cells. This phenomenon was later referred to as ‘agglutination’, and gave rise to the introduction of the term ‘agglutinin’ or phytohemagglutinin [2]. Research from Landsteiner and Raubitschek demonstrated that many lectins are nontoxic [3]. Because some plant agglutinins are able to distinguish between erythrocytes from different blood types, Boyd and Shapleigh [4] proposed the name ‘lectins’, from the Latin ‘legere’, meaning to pick out or select, and underlining the fact that these proteins target cells with some specificity, referring to the blood group specificity of several plant lectins [5, 6]. Watkins and Morgan [7] deciphered that this agglutination activity of lectins is related to the recognition of carbohydrate structures on the surface of erythrocytes, as deduced from the fact that particular carbohydrates and polysaccharides can inhibit the agglutination activity of lectins with red blood cells. Hapten inhibition studies highlighted the specific interaction between lectins and carbohydrate structures, thus proving that lectins possess carbohydrate-binding activity. These findings promoted the use of the term ‘lectins’, rather than ‘agglutinins’, since not all lectins can agglutinate cells. More details on the history of plant lectins can be found in some review papers [8, 9].
Concanavalin A from the seeds of jack bean (Canavalia ensiformis L.) was the first lectin that was purified to homogeneity [10]. The introduction of affinity chromatography in the late 1960s was an important step towards the purification of more carbohydrate-binding proteins [11], and resulted in the availability of lectins with higher purity, contributing to the biochemical characterization of the proteins, their physicochemical properties as well as their biological activities. Because of the high levels of lectins in legume seeds, lectin research focused on legume lectins for several decades. Consequently, the legume lectins are one of the best studied groups of lectins. The diversity, structural organization and biological activities of legume lectins have been reviewed recently [12,13,14].
Over the years the definition of lectins has been fine-tuned [15, 17,18,19,20]. Lectins are by definition “proteins that specifically recognize and bind to carbohydrate structures, but do not possess enzymatic activity”. Plant lectins harbor one or more carbohydrate-binding sites that will recognize carbohydrate structures, either endogenous (plant own) and/or exogenous (foreign, non-plant derived) glycan structures. Lectins interact with mono- or disaccharides, polysaccharides and more complex carbohydrate structures. Many years of lectin research have shown that glycoconjugates, including glycoproteins and glycolipids, are more likely to be the natural ligands for most lectins [8].
Plant lectins in vegetative storage tissues
When I entered the lectin field in the late ‘80 s, plant lectin research was mainly focused on the purification of lectins from seeds. Our attention was triggered by the fact that plant storage tissues, such as bark and bulbs, are a rich source of carbohydrate-binding proteins. The discovery of lectins in vegetative storage tissues gave a boost to plant lectinology and resulted in the purification and characterization of plant lectins with diverse molecular structures and carbohydrate-binding properties.
Our research team contributed largely to the identification and purification of bulb lectins from a variety of monocotyledonous plant families, including Amaryllidaceae (Galanthus nivalis L. (snowdrop), Narcissus pseudonarcissus L. (daffodil), Hippeastrum hybrid (amaryllis)), Orchidaceae (Listera ovata (L.) R. Br., twayblade), Liliaceae (Tulipa sp., tulip), Alliaceae (Allium sativum L.; garlic), Allium porrum L.; leek), Allium cepa L.; onion), Araceae (Arum maculatum L.; lords-and-ladies), Asparagaceae (Scilla campanulata L.; bluebell) [21,22,23,24,25,26,27]. These so-called monocot lectins attracted a lot of attention, especially because of their high specificity for mannose. Unlike Concanavalin A that recognizes both mannose and glucose, the Galanthus nivalis agglutinin (GNA) targets only mannose and specifically recognizes the terminal mannose groups in high-mannose N-glycans [28]. The unique specificity of GNA prompted the detailed analysis of its biological activities (insecticidal activity, antiviral activity) and boosted its use as a tool in glycobiology. Nowadays, GNA is the model lectin and represents a large group of so-called GNA-related lectins with high preference for mannose and mannose-containing oligosaccharides [29].
Our research on monocot mannose-binding bulb lectins was extended to lectins in a variety of vegetative storage tissues from dicotyledonous species, such as the bark lectins from Sambucus nigra L. (elderberry), Robinia pseudoacacia L. (black locust) and Morus nigra L. (mulberry) [30,31,32,33,34,35,36], and the rhizome lectins from Urtica dioica L. (stinging nettle) and Calystegia sepium L. (hedge bindweed) [37,38,39]. The characterization and structural analysis of the lectins from vegetative storage tissues disclosed the huge variety among lectins in terms of molecular structures and carbohydrate-binding specificities. In addition, we revealed the presence of many isolectins [33, 37, 40,41,42,43]. The occurrence of these isoforms prompted us to advance the lectin field and proceed from purification of lectins to molecular cloning of lectin genes or cDNA sequences, which allowed to unravel the mystery of isolectins. Most lectins are encoded by gene families, characterized by highly homologous sequences differing in a few amino acids, resulting in isoforms with slightly different isoelectric points, allowing to explain the multiple peaks observed after ion exchange chromatography of purified lectins [33, 41, 43].
Discovery of stress-inducible plant lectins
In the 1970s-1990s lectin research focused almost exclusively on lectins that were abundant in seeds or vegetative tissues. These lectins are now referred to as the ‘classical’ lectins to distinguish them from the stress-inducible lectins that were first described around the year 2000. Our research group was the first to report on stress-inducible lectins, in particular the salt stress-inducible lectin from rice (Oryza sativa L.) shoots [44] and the jasmonate-inducible lectin from tobacco (Nicotiana tabacum L.) leaves [45]. The latter lectins are expressed at very low levels and hardly detectable when plants are grown under normal growth conditions. However, lectin expression is upregulated when the plant is exposed to stress. Consequently, these lectins are designated as ‘stress-inducible’ plant lectins [46].
Interestingly, we could show that a diversity of plant lectin families harbors both classical and stress-inducible lectins, suggesting that both classes of lectins have evolved in parallel throughout evolution [46]. Though both the classical and the stress-inducible lectins do not show important differences in their three-dimensional structures, they locate to different compartments of the plant cell [47]. The low-abundant lectins are synthesized without a signal peptide and locate mainly to the cytoplasm, but some of these lectins can also enter into the nucleus. Consequently, the term ‘nucleocytoplasmic lectins’ was introduced [48]. In contrast, most classical lectins are synthesized with a signal peptide, and therefore follow the secretory route from the endoplasmic reticulum to the Golgi apparatus, and will finally locate to the vacuole, the plasma membrane or can be secreted to the cell wall and the extracellular space [49]. The novel concept of lectins being present in the nucleus also urged us to develop novel ideas related to functionality of these carbohydrate-binding proteins [50].
Diversity and classification of plant lectins
Although all lectins share the ability to specifically recognize and bind carbohydrate structures, these proteins have very different molecular structures, biochemical and biophysical properties, and therefore most likely are also implemented in diverse biological functions. In the past 130 years, hundreds of plant lectins have been identified and characterized in some detail [51], introducing the need for a classification system of lectins. Over the years, researchers have tried to establish different classification systems, aiming to organize the large and heterogeneous group of plant lectins considering their specificity for particular carbohydrate structures (e.g. mannose-binding lectins) or the plant family they were purified from (e.g. legume lectins). However, these classification systems did not succeed in obtaining more homogeneous groups of lectins.
Based on their molecular structure, we have divided the group of plant lectins in four classes, referred to as merolectins, hololectins, chimerolectins and superlectins. Merolectins contain a single lectin domain. In contrast, hololectins and superlectins contain multiple lectin domains. A superlectin contains several lectin domains with different carbohydrate-binding specificities. Chimerolectins are lectins composed of a lectin domain linked to one or more protein domains with another protein function [18].
Available data from protein sequencing as well as molecular cloning of cDNA sequences encoding lectins led us to elaborate a new classification system for plant lectins according to families of structurally related proteins. In 1998 the original classification system consisted of 7 plant lectin families [19], but in 2008 it was extended to 12 plant lectin families to accommodate the data on all newly identified carbohydrate-binding lectins from plants [49]. Lectin families are named after the first lectin of this family that was characterized in detail. In alphabetical order these are: the Agaricus bisporus agglutinin family, the Amaranthins, the chitinase-related agglutinins, the Cyanovirin domain lectins, the Euonymus-related lectins, the Galanthus nivalis agglutinin (GNA)-related lectins, the Hevein domain lectins, the Jacalin-related lectins, the legume lectins, the Lysin M (LysM) domain lectins, the Nicotiana tabacum agglutinin (Nictaba)-related lectins and the Ricin-B domain lectins. In addition, there are the lectins in the endoplasmic reticulum, such as calnexins, calreticulins and malectins. The latter lectins are important for protein folding and are also present in other kingdoms of life [52]. More details on the different plant lectin families have been described in some recent reviews [9, 20].
The molecular cloning of lectins facilitated the determination of the amino acid sequences of lectin polypeptides, which in turn progressed our knowledge on the three-dimensional structure of the lectins and their carbohydrate-binding sites. The diversity of plant lectin families prompted the search for the lectin motifs (short conserved sequence patterns associated with carbohydrate-binding activity) in each lectin family and deeper insight into the specificity of different lectins. As illustrated in Fig. 1, the structural diversity among plant lectins is large. The three-dimensional structure of most lectin domains contains a high content of β-sheets, and only few if any α-helices. These β-sheets will arrange into β-sandwich, β-trefoil, β-prism and barrel structures, depending on the lectin domain. Quaternary structures most often contain two or four subunits stabilized by hydrophobic interactions, hydrogen bonds and electrostatic interactions. The legume lectin domain typically contains metal ions (Mn2+ and Ca2+), but is the only lectin domain that requires metal ions for its activity. For more information we refer to the structural database for lectins and the Unilectin Web Platform (https://www.unilectin.eu/) [53, 54].
Three-dimensional structures representing different legume lectins, GNA-related lectins and Jacalin-related lectins. Graphs represent A Pisum sativum lectin (PSA) monomer (2LTN), B PSA dimer (2LTN), C Con A tetramer (1CVN), D GNA monomer (1MSA), E ASA dimer (1KJ1), F GNA tetramer (1MSA), G Scilla campanulata tetramer (1B2P), H Jacalin monomer (1JAC), I Calsepa dimer (1OUW), J Jacalin tetramer (1UGW), K Morniga-M tetramer (1XXQ), L Morniga-G tetramer (model), M Heltuba octamer (1C3K). PDB codes are given between brackets. Pictures were rendered using the ChimeraX software [154]
Since the classification in plant lectin families is based on the sequence of the lectin domain, all lectins within one family will have a similar fold. However, lectin domains within one family can be organized differently (forming monomers, dimers, tetramers, etc.) with one or more carbohydrate-binding sites per lectin domain (Fig. 1). Typically, a lectin domain contains one carbohydrate-binding site that will bind the carbohydrate structure non-covalently. Hydrogen bonds, electrostatic interactions and hydrophobic stacking (mainly with tryptophan residues) are involved in the specific binding of the carbohydrate structure [55]. The GNA-related lectins are unique in that they contain 3 carbohydrate-binding sites per lectin domain. X-ray crystallography revealed that the GNA monomer folds as a β-prism composed of three anti-parallel four-stranded β-sheets, forming the three binding sites. Since GNA is a tetramer, it contains 12 carbohydrate-binding sites in its quaternary structure, allowing the lectin domain to specifically recognize mannose oligosaccharides (Fig. 2) [56, 57].
Organisation of the carbohydrate-binding site in Lathyrus ochrus isolectin I (LoLI, A-D) and Galanthus nivalis lectin (GNA, E–H). A, B LoLI in complex with Mannose (Man, PDB code 1LOB). C, D LoLI in complex with a biantennary octasaccharide of the N-acetyllactosamine type from lactotransferrin (PDB code 1LOF). E, F GNA in complex with α-methylmannoside (Man, PDB code 1MSA). G, H GNA in complex with three mannosyl residues from a mannopentaose (PDB code 1JPC). Alpha and Beta refer to the alpha and beta chains composing LoLI. Hydrogen bonds connecting the carbohydrates to the amino acid residues forming the binding site are represented by dashed lines. The shallow depression on the lectin surface corresponding to the monosaccharide-binding site that can accommodate simple sugars is delineated by a yellow dashed line. The green and violet spheres represent the Ca2+ and Mn2+ ions that stabilize the carbohydrate-binding site of legume lectins. Pictures were rendered using the ChimeraX software [154]
In the early days, lectin research focused mainly on interactions with simple sugars (monosaccharides, disaccharides) or glycoproteins. The elaboration of new tools and more sensitive techniques (such as frontal affinity chromatography, surface plasmon resonance, glycan array technology) revealed that the pool of targeted carbohydrate structures for lectins is large and diverse, contributing to the many roles of lectins in plants and beyond. Specificity studies as well as the elucidation of three-dimensional structures in complex with carbohydrates have enabled to decipher the carbohydrate-binding specificity for many plant lectins and also pointed out the diversity and complexity among lectins. The specificity of the binding site is not linked to a plant lectin family. For instance, mannose-binding lectins have been retrieved in multiple plant lectin families, including legume lectins, GNA-related lectins and jacalin-related lectins [58,59,60].
Furthermore, there is no clear correlation between the lectin domain structure and its specificity. In legume lectin domains the folding of one particular loop determines the specificity, ranging from mannose/glucose-binding to Gal/GalNAc, fucose or sialic acid binding [12,13,14, 19, 61]. In addition, we have shown that subtle differences can be responsible for important changes in the specificity. For instance, proteolytic cleavage of the jacalin sequence into the α and β-subunits is important for galactose-binding activity of jacalin-related lectins. In mannose-binding jacalin-related lectins this cleavage is absent. However, structural analyses demonstrated that the carbohydrate-binding site of jacalin can accommodate different monosaccharides. Although jacalin is especially known for its binding to galactose and galactose-containing glycans, it can also interact with mannose, indicating that the β-prism structure of jacalin is flexible [35, 62,63,64,65].
Lectins will bind carbohydrate or glycan structures in their carbohydrate recognition domain. These lectin-monosaccharide interactions are typically relatively weak (in the millimolar range, 10–3-10–4 M). However, lectins exhibit a much higher affinity towards oligosaccharides and glycan structures (in the micromolar range, 10–6-10–7 M) either because of ligand multivalency or the presence of an extended binding site (Fig. 2) [66,67,68]. In most cases the lectins will target one or more terminal residues of complex carbohydrate structures. However, this is not always the case. The tobacco lectin Nictaba was first described as a (GlcNAc)n binding lectin based on hapten inhibition assays and its interaction with GlcNAc oligomers in surface plasmon resonance experiments [45]. However, detailed binding studies for Nictaba using glycan arrays also revealed its interaction with both high-mannose and complex N-glycans. Saturation difference NMR analyses allowed to unravel the molecular basis for the interaction between Nictaba and its targets, and was able to show that the carbohydrate-binding site of Nictaba can accommodate the Man3GlcNAc2 core of N-glycans as well as chitotriose [69, 70].
Over the years we have developed a special interest in mannose-binding lectins. Multiple lectin families with different structural scaffolds containing one or more carbohydrate-binding sites are involved in the specific recognition and binding of mannose and mannose-containing glycans. Overall, the mannose-binding site represents a rather small, central, carbohydrate-binding pocket that can accommodate a single mannose residue. However, this site is surrounded by an area, referred to as the extended binding site, responsible for the specific recognition of larger mannose-containing oligosaccharides or N-glycan chains, as shown in Fig. 2, for Lathyrus ochrus isolectin I (L.) DC. and GNA. Thus, the monosaccharide-binding site can be considered as part of the more extended oligosaccharide-binding site, allowing these lectins to interact with more complex glycans. From a physiological point of view, these more complex carbohydrate structures are more likely to be the targets for lectins in their natural environment, considering that the concentration of monosaccharides in cells and tissues is very low [60, 71]. More detailed information on the structural organization of plant lectins can be found in some recent papers [60, 72].
Lectin domains and evolutionary dynamics
In the last decades, a lot of sequence information became available from proteomics, genomics and transcriptomics analyses, and enhanced the knowledge related to three-dimensional structure of lectin domains as well as the evolutionary relationships between lectins. Our research group performed genome-wide searches for lectin motifs in multiple fully sequenced plant genomes, resulting in new ideas and concepts for plant lectins. First, our analysis of 38 genomes exposed that some lectin domains are highly conserved, ranging from algae to higher plants [20]. The strong conservation of several lectin motifs, such as Euonymus-related lectins, GNA-related lectins, heveins, legume lectin domains, LysM domains, Nictaba-related lectins, malectins and RicinB domains, highlights their significance for plants. Although some lectin domains are unique for plants, a few carbohydrate-binding domains are shared between plants, animals, fishes, bacteria and other kingdoms of life [20, 73,74,75,76,77]. For instance, the RicinB domain has been reported in bacteria, fungi, insects, fishes and in mammals [49].
Second, the genome-wide investigation on nucleotide sequences led to deduce the amino acid sequences and revealed that the majority of lectin sequences encode chimeric lectins (composed of one or more lectin domains coupled to another protein domain). In contrast, the classical lectins mainly belong to the class of hololectins, composed of lectin subunits/domains only. Our comparative study for some important model plants, including Arabidopsis thaliana L., Oryza sativa L. (rice), Cucumis sativus L. (cucumber) and Glycine max L. (soybean), uncovered that additional protein domains are recurrently been integrated into lectins in multiple plant species [77]. For instance, the F-box domain, kinase domain and glycosyl hydrolase domain have been identified in multiple chimerolectins and can occur in combination with different lectin domains [73,74,75,76,77]. The fact that the lectin domain can be linked to protein domains with other functions suggested that the carbohydrate-binding domain can be part of a protein with multiple functional domains and urged us to change our view related to the functionality of these lectins [20, 77].
Third, the extensive search for lectin motifs in multiple plant genomes for which complete sequences are available suggested that plant genomes contain a large battery of lectin sequences. As mentioned above, the majority of the lectin sequences encode multiple protein domains, including one or more lectin domains [77]. Furthermore, the sequences from most of these lectins have never been reported before. Transcriptome analyses from different experiments investigating expression under stress conditions further indicated that the transcript levels for most of the sequences are variable, and can be affected by a number of stress conditions, including abiotic (e.g. salt, drought, and heat) as well as biotic stresses (e.g. pathogens and insects).
The knowledge on lectins as multidomain proteins also adds to the complexity. It has become clear that through evolution lectin domains have been used as building blocks to create new chimeric proteins with multiple domains and with multiple activities [20, 79]. Considering that many lectin sequences also contain other functional protein domains, we have suggested to broaden the definition for lectins: “proteins can be considered lectins if they contain one or more lectin domains, or have a domain architecture including one or more lectin domains in combination with other protein domains” [79]. The lectin domain can bind carbohydrates or glycan structures in a specific and reversible way, but should be devoid of enzymatic activity.
Role of lectins in plants
Depending on their location in the cell, lectins will interact with different targets, either endogenous targets (i.e., the carbohydrate structures present inside the plant cell) or exogenous targets (i.e., the carbohydrates moieties on the surface of pathogens) [80]. Our discovery of lectins residing in the nucleus and in the cytoplasmic compartment(s) of the plant cell has changed the ideas on the physiology and biological functions of lectins drastically. In the past, lectins were viewed as storage proteins or proteins with no specific function in the cell unless protection against attack from herbivorous insects and pathogens. It is now considered that lectins are involved in many more biological processes related to stress signal transduction and defense, and can fulfill specific functions inside the plant cell or in the interaction with other organisms [18, 48, 49, 78,79,80].
The interest in ricin was originally sparked because of the toxicity of the protein. However, it is now clear that not all plant lectins are toxic proteins. In fact, plant lectins being ubiquitous in the plant kingdom, are part of our everyday diet. For example, lectins are present in many plant foods such as beans, potatoes, peas, cereals, tomatoes, garlic, banana, etc. Although some lectins are known to cause nutritional deficiencies and allergic reactions in humans, the concentration of lectins in most foods is far too low to pose a threat to healthy individuals [81, 82]. Furthermore, soaking and cooking will destroy the lectin in foods with a high lectin content, such as green beans [83, 84].
Being abundant proteins in seeds and vegetative tissues, lectins were first considered as storage proteins to provide the plant with a source of nitrogen. However, the observation that many plant tissues with high levels of lectin are not eaten by e.g. herbivores prompted us to put forward the hypothesis that lectins play a role in plant defense [18]. Hence, although not all lectins are toxic proteins, the ‘toxicity’ or deterrent activity of some of these proteins is still an important factor to explain the role of lectins as a part of plant defense. Using different experimental approaches, such as the addition of purified lectins to the artificial diet of insects or in the culture medium of fungi, bacteria or nematodes, as well as experiments with transgenic plants overexpressing lectins have proven that some lectins have deterrent activity or are toxic to several pathogens and insects. The role of lectins in plant defense has been reviewed extensively in recent years [85,86,87,88].
Most research illustrating the toxicity of lectins has been retrieved from studies with insects [85, 86]. The legume lectins and especially the group of GNA-related lectins have been studied in a greater detail for their insecticidal activity in different insect species. More recently, the stress-inducible jacalin-related and Nictaba-related lectins have been shown to possess toxic effects on insects [90,91,92]. Several transgenic crops overexpressing lectin genes have been investigated with the idea of using lectins as part of integrated insect pest control strategies [86, 89,90,91].
We and others have focused largely on the insecticidal activity of GNA and GNA-related lectins, such as the garlic lectin ASAL [93]. GNA attracted a lot of interest because it was the first lectin that was active on Hemiptera, being sucking insects, such as aphids [94]. Another interesting feature of GNA is that the lectin can be taken up by insect cells and tissues, and can cross the gut border [95]. Consequently, GNA and GNA-related lectins have been used as a carrier molecule to transport toxic proteins, such as neuropeptides, to reach the hemolymph of insects [96]. GNA and ASAL have been fused to toxins (e.g. the toxin from the South Indian red scorpion or the spider venom), enhancing the toxicity of these fusion proteins [96,97,98,99,100]. To circumvent resistance to evolve over time, transgenic lines expressing both lectins and Cry toxins have been developed for different crops. Stacking with Cry proteins will help to overcome problems related to resistance but at the same time will create transgenic lines resistant to both chewing and sucking insects [101, 102]. Obviously, the potential use of these transgenic crops is subject to investigation for their toxicity against higher animals. Feeding trials with rats for 90 days have reported no adverse effects from consumption of rice transformed with GNA [103, 104].
A lot of work aimed to decipher the mode of action of GNA-related lectins in insects. In general, lectins will be taken up by insects chewing on plant materials or sucking the phloem sap. Plant lectins will pass through the gastro-intestinal tract where they can bind with glycoproteins on epithelial cells [85, 86, 105]. In addition, toxicity of lectins is related to their ability to pass through the peritrophic matrix, depending on the molecular size of the lectin [106]. Overall, plant lectins are highly resistant to digestive enzymes in the insect. It has been shown that resistance to proteolytic degradation is an important parameter for insecticidal activity. The entomotoxic effects of lectins are often observed after lectin ingestion resulting in reduced larval weight, fecundity, delay in pupation and development, and in some cases mortality. In addition to reduction of growth, lectins can also disrupt midgut microvilli and provoke changes in transcript levels of several genes [107, 108]. Although lectin binding is a prerequisite for toxicity, uptake of lectins in the cell is not. Proteomics studies have further confirmed that lectin binding to cell surface glycoproteins can be the trigger for a signaling cascade, resulting in deterrent or toxic effects [85, 86, 108].
The binding of plant lectins to glycosylated proteins of insects being an important interaction to explain the insecticidal activity of lectins, prompted us to study in more detail the glycosylation in insects and the importance of glycosylation for insect development. Analyses of the N-glycomes from several insects revealed that high-mannose and paucimannose N-glycans are highly abundant, explaining why especially mannose-binding lectins show good interaction with insect glycoconjugates [109,110,111,112]. Recent research also indicated differences in the N-glycan profile for male and female insects of Nilparvata lugens, with male insects containing less high-mannose and more paucimannose type structures compared to the female insects [110, 111]. Both O- and N-glycosylation are important for insect physiology and development, as shown by RNAi experiments. Indeed, silencing of different enzymes important for the glycosylation process in insects caused failure of postembryonic development and wing formation [109, 113].
As mentioned above most lectin sequences encode chimeric proteins. At present little is known about the functionality of typical chimeric lectins, and the physiological importance of the lectin domain for the plant. One important group of chimeric lectins, known as lectin receptor kinases, is located on the plasma membrane and in the cell wall where they serve a role in the recognition of glycosylated molecules, such as those present on the surface of pathogens [88, 114, 115].
Lectins present in the nucleus and/or in the cytoplasm represent minor proteins. Their concentrations are variable and dependent on the plant response to abiotic and biotic stresses. Since lectin concentrations inside the cell are upregulated in response to environmental cues, these so-called stress-inducible lectins are considered to be part of plant signaling and stress responses [78, 116]. Multiple examples have shown the contribution of several stress-inducible lectins to plant immunity [117, 118].
Plant lectins as tools
Lectins from plants can be used for agricultural purposes. The knowledge that some carbohydrate-binding proteins can help the plant to cope with particular stress conditions can be exploited to the benefit of other plants lacking this type of lectin. Using a biotechnological approach, the expression of lectin genes can be altered, either overexpressed or blocked, or new genes for lectins can be introduced in the plant genome, aiming to improve the plant’s growth and development [119,120,121].
In addition, considering the specific interaction of lectins with glycosylated structures as well as the ability of some plant lectins to trigger immune responses or cell death, lectins can be used for biomedical research. Since their discovery plant lectins have been developed as important tools in the field of glycobiology, enabling to study the importance of protein-carbohydrate interactions. Lectins have been used for biomedical applications, ranging from diagnostics to cancer research [9, 122, 123]. A lot of information on lectins and cancer has been compiled in the database CancerLectinDB (http://proline.physics.iisc.ernet.in/cancerdb) [124]. Some of these applications are described here, highlighting primarily our contributions to the use of plant lectins as tools for glycobiology.
Lectins in the study of glycoproteins
It has been well-established that carbohydrate structures on the cell surface carry biological information. However, one of the major challenges is to decipher the glycocode and understand the importance of glycosylation which in turn can contribute to the future cure of diseases. Since plant lectins recognize and bind specific carbohydrate structures they can be exploited to detect specific glycans or changes in glycosylation occurring on cells or tissues. Our laboratory has contributed significantly to the purification and characterization of many plant lectins from natural sources. More recently, recombinant DNA technology has enabled the use of heterologous expression systems to make recombinant lectins. Taking advantage of the glycan array technology as provided by e.g. the Consortium for Functional Glycomics (http://www.functionalglycomics.org) detailed analyses of the carbohydrate-binding properties were performed allowing to put forward plant lectins with unique specificities. For example, GNA, the mannose-binding lectin from snowdrop (Galanthus nivalis) and SNA-I, the sialic acid binding agglutinin from elderberry (Sambucus nigra) turned out to be powerful tools [69]. Plant lectins with a wide variety of specificities have been included in lectin microarrays. The microarray panels consist of lectins immobilized on chips, allowing high throughput screening and easier detection of glycans in samples [125,126,127].
The interaction of lectins with glycan structures can inhibit cancer cells, in most cases by inducing autophagy and apoptosis. These antiproliferative and cytotoxic effects of particular lectins can help to fight cancer. For example, Morniga-M, a mannose-binding jacalin-related lectin from the bark of mulberry (Morus nigra), activates human resting T-lymphocytes and induces cell death of activated T cells. Morniga-G, the T/Tn-specific lectin from the bark of Morus nigra, induces the cell death of Tn-positive leukemic cells using O-glycosylation-, caspase-, and TRAIL/DR5-dependent pathways [128,129,130].
Lectins as delivery vehicles
Photochemotherapy can be used for the treatment of various tumors, and it has been suggested that photosensitizer efficiency can be enhanced by conjugation with plant lectins allowing specific targeting of photosensitizers towards aberrant glycosylation of tumor cells. In particular, the jacalin-related lectin Morniga-G with high affinity for tumor-associated T and Tn antigens was used to eliminate leukemic cells from the blood. The Morniga-G/photosensitizer conjugate was promising because it was poorly toxic when irradiated under white light, but showed strong phototoxicity when irradiated in the therapeutic window, thus preferentially killing cancerous lymphocytes [131, 132].
The fact that lectins can specifically bind glycosylated biomolecules makes them nice tools for targeted delivery of molecules. Nanoparticles decorated with lectins have been used to deliver molecules to cells where glycosylated proteins and lipids on the cell surface serve as binding sites for the lectins [133, 134]. Other examples for lectin mediated delivery involve the mannose-binding GNA. The snowdrop lectin is known to be toxic for aphids but is non-toxic to caterpillars [94]. Since GNA was shown to be transported to the hemolymph of insects after oral ingestion, the lectin domain can be used to create novel insecticides. As mentioned above fusion proteins consisting of a toxin domain linked to GNA were targeted to the hemolymph after oral uptake, and provoked insecticidal activity towards insects from different orders [96,97,98]. Recently, the snowdrop lectin was shown to accelerate the delivery of dsRNA in lepidopteran midgut cells [135]. GNA complexed dsRNA resulted in a faster and increased dsRNA uptake in midgut cells. In addition, the faster uptake correlated with an increased RNAi efficiency in these cells. Furthermore, feeding of Spodoptera exigua with GNA-complexed dsRNA yielded higher mortality compared to insects in the control group.
Lectins in the study of viral infections
Many plant lectins have been reported as antiviral proteins and have been suggested as possible candidates to prevent viral infections [136, 137]. Though several hurdles need to be overcome before lectins can be used in the clinic, several plant lectins have advanced our knowledge on viral biology, especially the enveloped viruses such as human immunodeficiency virus (HIV), influenza virus and coronaviruses that contain a highly glycosylated coat. The glycan structures on the surface proteins facilitate diverse structural and functional roles in the virus. The entry of the virus in the cell involves fusion between the viral membrane and the cellular membrane of the target cells, a process catalyzed by specific glycoproteins. Many soluble plant lectins bind to viral surface glycoproteins, and have served as nice tools to understand the infection process. Several mannose-binding lectins (e.g. GNA, Hippeastrum hybrid agglutinin HHA) and to a lesser extent GlcNAc binding lectins (e.g. Urtica dioica agglutinin UDA) have been reported to recognize HIV-1 virus particles and can interfere with virus replication in cell cultures. These lectins prevent syncytia formation between HIV infected cells and uninfected T-lymphocytes [138,139,140,141]. Lectins can prevent viral entry into cells, but probably also play a role in preventing HIV transmission, and therefore have been suggested as microbicide drugs [136, 141]. Exposure of HIV particles to GNA or HHA for a long time period yielded changes in the glycosylation profile for glycoprotein 120 (gp120), resulting mainly from mutations in the N-glycosylation sites (deletion of glycans) and occasionally the creation of new N-glycosylation sites (new glycans), compromising the infectivity of the virus [142, 143]. In addition, lectin selection for the virus particles creates holes in the glycan shield, enabling antibodies to reach the epitopes on the viral glycoproteins that were previously covered by glycans. In the case of gp120, most of these N-glycans are of the high-mannose type [136].
In contrast to HIV particles, coronaviruses contain more complex N-glycans. A screening with natural lectins revealed that some mannose-binding lectins, e.g. the GNA-related lectin from leek, exhibited the strongest anti-coronavirus activity. In addition, some galactose-, N-acetylgalactosamine-, glucose-, and N-acetylglucosamine-specific plant lectins also displayed anti-coronaviral properties. Two targets for binding early in the replication cycle and at the end of the infection cycle of SARS-CoV, respectively, have been identified [144].
Lectins with strong activity against HIV also showed strong antiviral activity against hepatitis C virus [145]. More recently, GNA, HHA and UDA have been reported to have anti-influenza virus activity against various influenza A(H1N1), A(H3N2), and B viruses in vitro. It is hypothesized that these lectins interact with N-glycans on the hemagglutinin in the envelope, since mutation of the N-glycan sites yielded a reduced activity against these mutant influenza viruses [146].
Interestingly, the GNA-related lectins, such as GNA and HHA qualify as potent inhibitors for HIV, hepatitis C virus, coronaviruses as well as influenza viruses in vitro. At present, lectins have not been investigated from a therapeutic viewpoint, but rather serve as interesting molecules to unravel viral biology. Since lectins are proteins, they can elicit an antibody response. In addition, it is not clear whether lectins can discriminate between cellular and viral glycans. Therefore, more research is required to investigate the potential use of lectins as therapeutics.
Lectins as decoders of the glycome
The cell stores a lot of information in its nucleus under the form of nucleic acids, the genetic code (DNA) or the transcripts (RNA), that will be functional at a given time in specific cells or tissues. The proteins are the work horses of the cell and play a key role in processes such as growth, development, metabolism, and replication. Enzymes in particular are needed to safeguard the well-functioning of a cell or organism. Among proteins, lectins represent a special group because of their ability to specifically recognize and bind to a diversity of carbohydrate structures. Many of these glycans are attached either to proteins and/or to lipids. Proteins ‘reading’ the glycans, the lectins, have revealed the dynamic regulation of glycosylation, both in time and in space. These data support the idea of a ‘sugar code’, the glycome, and lectins being the readers or decoders [147, 148]. In contrast to the proteins, the glycans are not encoded in the genome, making it challenging to study their presence and functionality in biological systems. Indisputably, the glycans are essential for diverse processes, ranging from proper folding to biological activity of proteins. Recently, the discovery of glycoRNAs, small noncoding RNAs carrying N-glycan modifications present mainly at the cell surface has added an additional level of complexity, and most likely an additional level of regulation of biological processes [149].
Lectins on one hand can read the ‘glyco’code, as is the case in applications where lectins are being used to decipher specific glycostructures (in particular the use of lectins in cytochemistry, histochemistry or as ligands for affinity-based chromatography). On the other hand, it is also clear that lectin-carbohydrate interactions can be the starting point for a signaling cascade, as in many immune responses [80, 116, 148].
It can be hypothesized that especially the lectins in the cytoplasm and in the nucleus will act as decoders. For example, the stress-inducible lectin from tobacco, called Nictaba, is present in the nucleus and was shown to interact with O-GlcNAc modified histone proteins. It is therefore reasonable to assume that under stress conditions Nictaba may modulate gene expression using chromatin remodeling [150,151,152].
Concluding remarks
Since the discovery of the first lectin, the field of lectinology has changed enormously. Whereas the research was focused on the identification of new plant lectins with interesting carbohydrate-binding properties for a very long time, it has now moved towards deciphering the biological role of lectins in plants as well as in many other organisms. At the same time the knowledge on the carbohydrate-binding properties of lectins and the identification of new lectins enabled the use of lectins as tools and facilitated or even boosted more applied research.
Over the years, the complexity of lectins has increased continuously. Lectins being located in diverse plant compartments added to this complexity. But especially our findings that the majority of lectin sequences encode chimeric proteins in which the lectin domain is only a small part of a larger multidomain protein urges to reconsider the functionality of lectins. Furthermore, the interaction between different protein domains and possible synergistic effects resulting from the presence of multiple protein domains should be investigated.
Recent studies have shown that glycans can severely impact plant growth and development but are also important for cellular signaling, plant immunity and stress tolerance [153]. Similarly, glycans in animal systems can be recognized by plant lectins, enabling the use of lectins as glycoprobes and useful tools for cancer diagnosis and therapy [126,127,128]. More detailed knowledge on the natural lectins or the introduction of protein engineering to make synthetic lectins will advance the field in coming years. Undoubtedly, further elucidation of lectin-glycan interactions as well as the biological processes relying on these interactions and signaling cascades can be the subject of exciting glycobiology and lectin research in a significant part of the twenty-first century.
References
Stillmark, H.: Über Ricin ein giftiges Ferment aus den Samen von Ricinus communis L. und einige anderen Euphorbiaceen. MD Thesis, University of Dorpat, Dorpat, Estonia (1888)
Elfstrand, M.: Über blutkörperchenagglutinierende Eiweisse. In: Görberdorfer Veröffentlichungen a. Band I., pp. 1–159 (1898)
Landsteiner, K., Raubitschek, H. Beobachtungen über Hämolyse und Hämagglutination. Zentralbl. Bakteriol. Parasitenk. Infektionskr. Hyg. Abt. 1: Orig. 45, 660–667 (1907)
Boyd, W.C., Shapleigh, E.: Specific precipitating activity of plant agglutinins (lectins). Science 119, 419 (1954)
Boyd, W.C., Reguera, R.M.: Hemagglutinating substances for human cells in various plants. J. Immunol. 62, 333–339 (1949)
Renkonen, K.O.: Studies on hemagglutinins present in seeds of some representatives of the family of Leguminoseae. Ann. Med. Exp. Biol. Fenn. 26, 66–72 (1948)
Watkins, W.M., Morgan, W.T.J.: Neutralization of the anti–H agglutinin in eel serum by simple sugars. Nature 169, 825–826 (1952)
Van Damme, E.J.M.: History of plant lectin research. Methods Mol. Biol. 1200, 3–13 (2014)
Tsaneva, M., Van Damme, E.J.M.: 130 years of Plant Lectin Research. Glycoconj. J. 37, 533–551 (2020)
Sumner, J.B., Howell, S.F.: The identification of the hemagglutinin of the jack bean with concanavalin A. J. Bacteriol. 32, 227–237 (1936)
Baues, R.J., Gray, G.R.: Lectin purification on affinity columns containing reductively aminated disaccharides. J. Biol. Chem. 252, 57–60 (1977)
Cavada, B.S., Osterne, V.J.S., Oliveira, M.V., Pinto-Junior, V.R., Silva, M.T.L., Bari, A.U., Lima, L.D., Lossio, C.F., Nascimento, K.S.: Reviewing Mimosoideae lectins: A group of under explored legume lectins. Int. J. Biol. Macromol. 154, 159–165 (2020)
Cavada, B.S., Pinto-Junior, V.R., Osterne, V.J.S., Oliveira, M.V., Lossio, C.F., Silva, M.T.L., Bari, A.U., Lima, L.D., Souza-Filho, C.H.D., Nascimento, K.S.: Comprehensive review on Caelsalpinioideae lectins: From purification to biological activities. Int. J. Biol. Macromol. 162, 333–348 (2020)
Cavada, B.S., Pinto-Junior, V.R., Oliveira, M.V., Osterne, V.J.S., Lossio, C.F., Nascimento, K.S.: A review of Vicieae lectins studies: End of the book or a story in the writing? Int. J. Biol. Macromol. 181, 1104–1123 (2021)
Goldstein, I.J., Hughes, R.C., Monsigny, M., Osawa, T., Sharon, N.: What should be called a lectin? Nature 285, 66 (1980)
Dixon, H.B.F.: Defining a lectin. Nature. 292, 192 (1981)
Barondes, S.H.: Bifunctional properties of lectins: lectins redefined. Trends Biochem. Sci. 13, 480–482 (1988)
Peumans, W.J., Van Damme, E.J.M.: Lectins as plant defense proteins. Plant Physiol. 109, 347–352 (1995)
Van Damme, E.J.M., Peumans, W.J., Barre, A., Rougé, P.: Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 17, 575–692 (1998)
Van Holle, S., Van Damme, E.J.M.: Messages from the past: new insights in plant lectin evolution. Front. Plant Sci. 10, 36 (2019)
Van Damme, E.J.M., Allen, A.K., Peumans, W.J.: Isolation and characterization of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett. 215, 140–144 (1987)
Van Damme, E.J.M., Allen, A.K., Peumans, W.J.: Related mannose-specific lectins from different species of the family Amaryllidaceae. Physiol. Plant. 73, 52–57 (1988)
Van Damme, E.J.M., Allen, A.K., Peumans, W.J.: Leaves of the orchid twayblade (Listera ovata) contain a mannose-specific lectin. Plant Physiol. 85, 566–569 (1987)
Van Damme, E.J.M., Peumans, W.J.: Developmental changes and tissue distribution of lectin in Tulipa. Planta 178, 10–18 (1989)
Kaku, H., Van Damme, E.J.M., Peumans, W.J., Goldstein, I.J.: New mannose-specific lectins from garlic (Allium sativum) and ramsons (Allium ursinum) bulbs. Carbohydrate Res. 229, 347–353 (1992)
Van Damme, E.J.M., Goossens, K., Smeets, K., Van Leuven, F., Verhaert, P., Peumans, W.J.: The major tuber storage protein of Araceae species is a lectin: Characterization and molecular cloning of the lectin from Arum maculatum L. Plant Physiol. 107, 1147–1158 (1995)
Wright, L.M., Van Damme, E.J.M., Barre, A., Allen, A.K., Van Leuven, F., Reynolds, C.D., Rougé, P., Peumans, W.J.: Isolation, characterization, molecular cloning and molecular modelling of two lectins of different specificities from bluebell (Scilla campanulata) bulbs. Biochem. J. 340, 299–308 (1999)
Shibuya, N., Goldstein, I.J., Van Damme, E.J.M., Peumans, W.J.: Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J. Biol. Chem. 263, 728–734 (1988)
Kaku, H., Van Damme, E.J.M., Peumans, W.J., Goldstein, I.J.: Carbohydrate binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch. Biochem. Biophys. 279, 298–304 (1990)
Peumans, W.J., Nsimba-Lubaki, M., Broekaert, W.F., Van Damme, E.J.M.: Are bark lectins of elderberry (Sambucus nigra) and black locust (Robinia pseudoacacia) storage proteins? In Molecular Biology of Seed Storage Proteins and Lectins. (L.M. Shannon and M.J. Chrispeels, eds). ASPP; Proceedings of the 9th annual symposium in plant physiology, UCR Riverside, pp. 53–63 (1986)
Peumans, W.P., Kellens, T.C., Allen, A.K., Van Damme E.J.M.: Isolation and characterization of a seed lectin from elderberry (Sambucus nigra L.) and its relationship to the bark lectins. Carbohydrate Res. 213, 7–17 (1991)
Van Damme, E.J.M., Barre, A., Rougé, P., Van Leuven, F., Peumans, W.J.: The NeuAc (α2,6)-Gal/GalNAc binding lectin from elderberry (Sambucus nigra) bark, a type 2 ribosome inactivating protein with an unusual specificity and structure. Eur. J. Biochem. 235, 128–137 (1996)
Van Damme, E.J.M., Barre, A., Smeets, K., Torrekens, S., Van Leuven, F., Rougé, P., Peumans, W.J.: The bark lectin of Robinia pseudoacacia contains a complex mixture of isolectins. Characterization of the proteins and the cDNA clones. Plant Physiol. 107, 833–843 (1995)
Van Damme, E.J.M., Barre, A., Rougé, P., Van Leuven, F., Peumans, W.J.: The seed lectins of black locust (Robinia pseudoacacia) are encoded by two genes which differ from the bark lectin genes. Plant Mol. Biol. 29, 1197–1210 (1995)
Rougé, P., Peumans, W.J., Barre, A., Van Damme, E.J.M.: A structural basis for the difference in specificity between the two jacalin-related lectins from mulberry (Morus nigra) bark. Biochem. Biophys. Res. Commun. 304, 91–97 (2003)
Wu, A.M., Wu, J.H., Singh, T., Chu, K.-C., Peumans, W.J., Rougé, P., Van Damme, E.J.M.: A novel lectin (Morniga M) from mulberry (Morus nigra) bark recognizes oligomannosyl residues in N-glycans. J. Biomed. Sci. 11, 874–885 (2004)
Van Damme, E.J.M., Broekaert, W.F., Peumans, W.J.: The Urtica dioica agglutinin is a complex mixture of isolectins. Plant Physiol. 86, 598–601 (1988)
Van Damme, E.J.M., Barre, A., Verhaert, P., Rougé, P., Peumans, W.J.: Molecular cloning of the mitogenic mannose/maltose-specific rhizome lectin from Calystegia sepium. FEBS Lett. 397, 352–356 (1996)
Peumans, W.J., Winter, H.C., Bemer, V., Van Leuven, F., Goldstein, I.J., Truffa-Bachi, P., Van Damme, E.J.M.: Isolation of a novel plant lectin with an unusual specificity from Calystegia sepium. Glycoconjugate J. 14, 259–265 (1997)
Van Damme, E.J.M., Kaku, H., Perini, F., Goldstein, I.J., Peeters, B., Yagi, F., Decock, B., Peumans, W.J.: Biosynthesis, primary structure and molecular cloning of snowdrop (Galanthus nivalis L.) lectin. Eur. J. Biochem. 202, 23–30 (1991)
Van Damme, E.J.M., Declercq, N., Claessens, F., Hemschoote, K., Peeters, B., Peumans, W.J.: Molecular cloning and characterization of multiple isoforms of the snowdrop (Galanthus nivalis L.) lectin. Planta. 186, 35–43 (1991)
Van Damme, E.J.M., Peumans, W.J.: Isolectins in Narcissus: complexity, inter- and intraspecies differences and developmental control. Physiol. Plant. 79, 1–6 (1990)
Does, M.P., Ng, D.K., Dekker, H.L., Peumans, W.J., Houterman, P.M., Van Damme, E.J.M., Cornelissen, B.J.C.: Characterization of Urtica dioica agglutinin isolectins and the encoding gene family. Plant Mol. Biol. 39, 335–347 (1999)
Zhang, W., Peumans, W.J., Barre, A., Houles-Astoul, C., Rovira, P., Rougé, P., Proost, P., Truffa-Bachi, P., Jalali, A.A.H., Van Damme, E.J.M.: Isolation and characterization of a jacalin-related mannose-binding lectin from salt-stressed rice (Oryza sativa) plants. Planta 210, 970–978 (2000)
Chen, Y., Peumans, W.J., Hause, B., Bras, J., Kumar, M., Proost, P., Barre, A., Rougé, P., Van Damme, E.J.M.: Jasmonic acid methyl ester induces the synthesis of a cytoplasmic/nuclear chitooligosaccharide-binding lectin in tobacco leaves. FASEB J. 16, 905–907 (2002)
Van Damme, E.J.M., Barre, A., Rougé, P., Peumans, W.J.: Cytoplasmic/nuclear plant lectins: a new story. Trends Plant Sci. 9, 484–489 (2004)
Peumans, W.J., Hause, B., Van Damme, E.J.M.: The galactose-binding and mannose-binding jacalin-related lectins are located in different subcellular compartments. FEBS Lett. 477, 186–192 (2000)
Lannoo, N., Van Damme, E.J.M.: Nucleocytoplasmic plant lectins. Biochim. Biophys. Acta. 1800, 190–201 (2010)
Van Damme, E.J.M., Lannoo, N., Peumans, W.J.: Plant lectins. Adv. Bot. Res. 48, 107–209 (2008)
Van Damme, E.J.M., Lannoo, N., Fouquaert, E., Peumans, W.J.: The identification of inducible cytoplasmic/nuclear carbohydrate-binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconjugate J. 20, 449–460 (2004)
Van Damme, E.J.M., Peumans, W.J., Pusztai, A., Bardocz, S.: Handbook of Plant Lectins: Properties and Biomedical Applications. John Wiley & Sons, Chichester, U.K. (ISBN 0–471–96445-X) (1998)
Tannous, A., Pisoni, G.B., Hebert, D.N., Molinari, M.: N-linked sugar-regulated protein folding and quality control in the ER. Sem. Cell Dev. Biol. 41, 79–89 (2015)
Bonnardel, F., Mariethoz, J., Salentin, S., Robin, X., Schroeder, M., Perez, S., Lisacek, F., Imberty, A.: UniLectin3D, a database of carbohydrate binding proteins with curated information on 3D structures and interacting ligands. Nucleic Acids Res. 47(D1), D1236–D1244 (2019)
Bonnardel, F., Perez, S., Lisacek, F., Imberty, A.: Structural Database for Lectins and the UniLectin Web Platform. Methods Mol. Biol. 2132, 1–14 (2020)
Rini, J.M.: Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 24, 551–577 (1995)
Hester, G., Kaku, H., Goldstein, I.J., Wright, C.S.: Structure of mannose-specific snowdrop (Galanthus nivalis) lectin is representative of a new plant lectin family. Nat Struct. Biol. 2, 472–479 (1995)
Hester, G., Wright, C.S.: The mannose-specific bulb lectin from Galanthus nivalis (snowdrop) binds mono- and dimannosides at distinct sites. Structure analysis of refined complexes at 2.3 Å and 3.0 Å resolution. J. Mol. Biol. 262, 516–531 (1996)
Barre, A., Van Damme, E.J.M., Peumans, W.J., Rougé, P.: Structure-function relationship of monocot mannose-binding lectins. Plant Physiol. 112, 1531–1540 (1996)
Barre, A., Bourne, Y., Van Damme, E.J.M., Peumans, W.J., Rougé, P.: Mannose-binding plant lectins: different structural scaffolds for a common sugar-recognition process. Biochimie 83, 645–651 (2001)
Barre, A., Bourne, Y., Van Damme, E.J.M., Rougé, P.: Overview of the structure-function relationships of mannose-specific lectins from plants, algae and fungi. Int. J. Mol. Sci. 20, 254 (2019)
Loris, R., Hamelryck, T., Bouckaert, J., Wyns, L.: Legume lectin structure. Biochim. Biophys. Acta 1383, 9–36 (1998)
Bourne, Y., Houlès Astoul C., Zamboni, V., Peumans, W.J., Menu-Bouaouiche, L., Van Damme, E.J.M., Barre, A., Rougé, P.: Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem. J. 364, 173–179 (2002)
Houlès Astoul, C., Peumans, W.J., Van Damme, E.J.M., Barre, A., Bourne, Y., Rougé, P.: The size, shape and specificity of the sugar-binding site of the jacalin-related lectins is profoundly affected by the proteolytic cleavage of the subunits. Biochem. J. 367, 817–824 (2002)
Barre, A., Peumans, W.J., Rossignol, M., Borderies, G., Culerrier, R., Van Damme, E.J.M.: Rougé, P. Artocarpin is a polyspecific jacalin-related lectin with a monosaccharide preference for mannose. Biochimie. 86, 685–691 (2004)
Rabijns, A., Barre, A., Van Damme, E.J.M., Peumans, W.J., De Ranter, C.J., Rougé, P.: Structural analysis of the polyspecific jacalin-related lectin MornigaM from the black mulberry (Morus nigra) in complex with mannose. FEBS J. 272, 3725–3732 (2005)
Hendrickson, O.D., Zherdev, A.V.: Analytical Application of Lectins. Crit. Rev. Anal. Chem. 48, 279–292 (2018)
Lee, R.T., Lee, Y.C.: Affinity enhancement by multivalent lectin-carbohydrate interaction. Glycoconj J. 17, 543–551 (2000)
Dam, T.K., Brewer, C.F.: Multivalent lectin-carbohydrate interactions energetics and mechanisms of binding. Adv. Carbohydr. Chem. Biochem. 63, 139–164 (2010)
Van Damme, E.J.M., Smith, D.F., Cummings, R., Peumans, W.J.: Glycan arrays to decipher the specificity of plant lectins. In: The Molecular Immunology of Complex Carbohydrates-3. Ed. A.M. Wu, Springer, pp. 841–854 (ISBN: 978–1–4419–7876–9) (2011)
Delporte, A., Van Holle, S., Lannoo, N., Van Damme, E.J.M.: The tobacco lectin, prototype of the family of Nictaba-related proteins. Curr. Prot. Pept. Sci. 16, 5–16 (2015)
Peumans, W.J., Barre, A., Hao, Q., Rougé, P., Van Damme, E.J.M.: Higher plants developed structurally different motifs to recognize foreign glycans. Trends Glycosci. Glycotechnol. 12, 83–101 (2000)
Surya, P.H., Deepti, M., Elyas, K.K.: Plant lectins: Sugar-binding properties and Biotechnological applications. S.T. Sukumaran, S.T., Sugathan, S., Abdulhameed, S. (eds.), Plant Metabolites: Methods, Applications and Prospects, Springer Nature Singapore Pte Ltd. (2020)
Dang, L., Van Damme, E.J.M.: Genome-wide identification and domain organization of lectin domains in cucumber. Plant Physiol. Biochem. 108, 165–176 (2016)
Van Holle, S., Van Damme, E.J.M.: Distribution and evolution of the lectin family in soybean (Glycine max). Molecules 20, 2868–2891 (2015)
Saeed, B., Baranwal, V.K., Khurana, P.: Identification and expression profiling of the lectin gene superfamily in mulberry. Plant Genome 9 (2016)
Eggermont, L., Verstraeten, B., Van Damme, E.J.M.: Genome–wide screening for lectin motifs in Arabidopsis thaliana. Plant Genome 10 (2017)
Van Holle, S., De Schutter, K., Eggermont, L., Tsaneva, M., Dang, J., Van Damme, E.J.M.: Comparative study of lectin domains in model species: New insights into evolutionary dynamics. Int. J. Mol. Sci. 18, 1136 (2017)
De Schutter, K., Tsaneva, M., Kulkarni, S.R., Rougé, P., Vandepoele, K., Van Damme, E.J.M.: Evolutionary relationships and expression analysis of EUL domain proteins in rice (Oryza sativa). Rice 10, 26 (2017)
Van Holle, S., Van Damme, E.J.M.: Signaling through plant lectins: modulation of plant immunity and beyond. Biochem. Soc. Trans. 46, 217–233 (2018)
Van Damme, E.J.M.: Carbohydrates in health and disease: a plant’s perspective. In: Glycome: The hidden Code in Biology. Nova Science Publisher, Dipak K Banerjee, editor; ISBN: 978–1–53619–377–0, pp. 201–216 (2021)
Peumans, W.J., Van Damme, E.J.M.: Prevalence, biological activity and genetic manipulation of lectins in foods. Trends Food Sci. Technol. 7, 132–138 (1996)
Barre, A., Van Damme, E.J.M., Simplicien, M., Benoist, H., Rougé, P.: Are dietary lectins relevant allergens in plant food allergy? Foods 9, 1724 (2020)
Luo, Y.-W., L., Xie, W.-H.: Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia faba L.). CyTA-J. Food 11, 43–49 (2013)
Shi, L., Arntfield, S.D., Nickerson, M.: Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 107, 660–668 (2018)
Vandenborre, G., Smagghe, G., Van Damme, E.J.M.: Plant lectins as defense proteins against phytophagous insects. Phytochem. 72, 1538–1550 (2011)
Macedo, M.L.R., Oliveira, C.F., Oliveira, C.T.: Insecticidal activity of plant lectins and potential application in crop protection. Molecules 20, 2014–2033 (2015)
Coelho, L.C.B.B., Silva, P.M.S., Oliveira, W.F., Moura, M.C., Pontual, E.V., Gomes, E.V., Paiva, P.M.G., Napoleao, T.H., Correia, M.T.S.: Lectins as antimicrobial agents. J. Appl. Microbiol. 125, 1238–1252 (2018)
Lannoo, N., Van Damme, E.J.M.: Lectin domains at the frontiers of plant defense. Front. Plant Sci. 5, 397 (2014)
Lagarda-Diaz, I., Guzman-Partida, A.M., Vazquez-Moreno, L.: Legume lectins: proteins with diverse applications. Int. J. Mol. Sci. 18, 1242 (2017)
Michiels, K., Van Damme, E.J.M., Smagghe, G.: Plant-insect interactions: what can we learn from plant lectins? Arch. Insect Biochem. Physiol. 73, 193–212 (2010)
Al Atalah, B., Smagghe, G., Van Damme, E.J.M.: Orysata, a jacalin-related lectin from rice, could protect plants against biting-chewing and piercing-sucking insects. Plant Sci. 221–222, 21–28 (2014)
Sadeghi, A., Smagghe, G., Broeders, S., Hernalsteens, J.P., De Greve, H., Peumans, W.J., Van Damme, E.J.M.: Ectopically expressed leaf and bulb lectins from garlic (Allium sativum L.) protect transgenic tobacco plants against cotton leafworm (Spodoptera littoralis). Transgenic Res. 17, 9–18 (2008)
Vandenborre, G., Groten, K., Smagghe, G., Lannoo, N., Baldwin, I.T., Van Damme, E.J.M.: Nicotiana tabacum agglutinin is active against Lepidopteran pest insects. J. Exp. Bot. 61, 1003–1014 (2010)
Hilder, V.A., Powell, K.S., Gatehouse, A.M.R., Gatehouse, J.A., Gatehouse, L.N., Shi, Y., Hamilton, W.D.O., Merryweather, A., Newell, C., Timans, J.C., Peumans, W.J., Van Damme, E.J.M., Boulter, D.: Expression of snowdrop lectin in transgenic tobacco plants results in added protection against aphids. Transgenic Res. 4, 18–25 (1995)
Fitches, E., Woodhouse, S.D., Edwards, J.P., Gatehouse, J.A.: In vitro and in vivo binding of snowdrop (Galanthus nivalis agglutinin; GNA) and jackbean (Canavalia ensiformis; Con A) lectins within tomato moth (Lacanobia oleracea) larvae; mechanisms of insecticidal action. J. Insect Physiol. 47, 777–787 (2001)
Fitches, E., Edwards, M.G., Mee, C., Grishin, E., Gatehouse, A.M., Edwards, J.P., Gatehouse, J.A.: Fusion proteins containing insect-specific toxins as pest control agents: snowdrop lectin delivers fused insecticidal spider venom toxin to insect haemolymph following oral ingestion. J. Insect Physiol. 50, 61–71 (2004)
Fitches, E.C., Bell, H.A., Powell, M.E., Back, E., Sargiotti, C., Weaver, R.J., Gatehouse, J.A.: Insecticidal activity of scorpion toxin (ButaIT) and snowdrop lectin (GNA) containing fusion proteins towards pest species of different orders. Pest Manag Sci. 66, 74–83 (2010)
Pham Trung, N., Fitches, E., Gatehouse, J.A.: A fusion protein containing a lepidopteran-specific toxin from the South Indian red scorpion (Mesobuthus tamulus) and snowdrop lectin shows oral toxicity to target insects. BMC Biotechnol. 6, 18 (2006)
Bhatti, M.U., Riaz, S., Toufiq, N., Adeyinka, O.S., Khan, A., Yousaf, I.? Tariq, M., Murtaza, S., Nasir, I.A., Tabassum, B.: The potential and efficacy of Allium sativum leaf lectin (ASAL) against sap-sucking insect pests of transgenic maize. Biologia 75, 2351–2358 (2020)
Boddupally, D., Tamirisa, S., Gundra, S.R., Vudem, D.R., Khareedu, V.R.: Expression of hybrid fusion protein (Cry1Ac::ASAL) in transgenic rice plants imparts resistance against multiple insect pests. Sci. Rep. 8, 8458 (2018)
Bharathi, Y., Vijaya Kumar, S., Pasalu, I.C., Balachandran, S.M., Reddy, V.D., Rao, K.V.: Pyramided rice lines harbouring Allium sativum (ASAL) and Galanthus nivalis (GNA) lectin genes impart enhanced resistance against major sap-sucking pests. J. Biotechnol. 152, 63–71 (2011)
Din, S.U., Azam, S., Rao, A.Q., Shad, M., Ahmed, M., Gul, A., Latif, A., Ali, M.A., Husnain, T., Shahid, A.A.: Development of broad-spectrum and sustainable resistance in cotton against major insects through the combination of Bt and plant lectin genes. Plant Cell Rep. 40, 707–721 (2021)
Poulsen, M., Kroghsbo, S., Schrøder, M., Wilcks, A., Jacobsen, H., Miller, A., Frenzel, T., Danier, J., Rychlik, M., Shu, Q., Emami, K., Sudhakar, D., Gatehouse, A., Engel, K.H., Knudsen, I.: A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol. 45, 350–363 (2007)
Poulsen, M.: Assessing biosafety of GM plants containing lectins. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 5. https://doi.org/10.1079/PAVSNNR20105034. (2010)
Walski, T., De Schutter, K., Cappelle, K., Van Damme, E.J.M., Smagghe, G.: Distribution of glycan motifs at the surface of midgut cells in the cotton leafworm (Spodoptera littoralis) demonstrated by lectin binding. Front. Physiol. 8, 1020 (2017)
Walski, T., Van Damme, E.J.M., Smagghe, G.: Penetration of peritrophic matrix is a key to lectin toxicity against Tribolium castaneum. J. Insect Physiol. 70, 94–101 (2014)
Gatehouse, A.M., Gatehouse, J.A., Bharathi, M., Spence, J., Powell, K.S.: Immunohistochemical and developmental studies to elucidate the mechanism of action of the snowdrop lectin on the rice brown planthopper, Nilaparvata lugens (Stal). J. Insect Physiol. 44, 529–539 (1998)
Shukle, R.H., Subramanyam, S., Williams, C.E.: Effects of antinutrient proteins on Hessian fly (Diptera: Cecidomyiidae) larvae. J. Insect Physiol. 58, 41–48 (2012)
Walski, T., Van Damme, E.J.M., Smargiasso, N., Christiaens, O., De Pauw, E., Smagghe, G.: Protein N-glycosylation and N-glycan trimming are required for postembryonic development of the pest beetle Tribolium castaneum. Sci. Rep. 6, 35151 (2016)
Scheys, F., De Schutter, K., Shen, Y., De Pauw, E., Yu, N., Smargiasso, N., Van Damme, E.J.M., Smagghe, G.: The N-glycome of the hemipteran pest insect Nilaparvata lugens reveals unexpected sex differences. Insect Biochem. Mol. Biol. 107, 39–45 (2019)
Scheys, F., Van Damme, E.J.M., Pauwels, J., Staes, A., Gevaert, K., Smagghe, G.: N-glycosylation site analysis reveals sex-related differences in protein N-glycosylation in the rice brown planthopper (Nilaparvata lugens). Mol. Cell. Proteom. 19, 529–539 (2020)
Liu, D., De Schutter, K., Smargiasso, N., De Pauw, E., Van Damme, E.J.M., Smagghe, G.: The N-glycan profile of the peritrophic membrane in the Colorado potato beetle larva (Leptinotarsa decemlineata). J. Insect Physiol. 115, 27–32 (2019)
Li, W., De Schutter, K., Van Damme, E.J.M., Smagghe, G.: RNAi-mediated silencing of pgants shows core 1 O-glycans are required for pupation in Tribolium castaneum. Front. Physiol. 12, 629682 (2021)
Vaid, N., Pandey, P.K., Tuteja, N.: Genome-wide analysis of lectin receptor-like kinase family from Arabidopsis and rice. Plant Mol. Biol. 80, 365–388 (2012)
Bellande, K., Bono, J.J., Savelli, B., Jamet, E., Canut, H.: Plant Lectins and Lectin Receptor-Like Kinases: How Do They Sense the Outside? Int. J. Mol. Sci. 18, 1164 (2017)
De Schutter, K., Van Damme, E.J.M.: Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules 20, 9029–9053 (2015)
Lambin, J., Demirel Asci, S., Dubiel, M., Tsaneva, M., Verbeke, I., Wytynck, P., De Zaeytijd, J., Smagghe, G., Subramanyam, K., Van Damme, E.J.M.: OsEUL lectin gene expression in rice: Stress regulation, subcellular localization and tissue specificity. Front. Plant Sci. 11, 185 (2020)
Al Atalah, B., De Vleesschauwer, D., Xu, J., Fouquaert, E., Höfte, M., Van Damme, E.J.M.: Transcriptional behavior of EUL-related rice lectins towards important abiotic and biotic stresses. J. Plant Physiol. 171, 986–992 (2014)
Eggermont, L., Stefanowicz, K., Van Damme, E.J.M.: Nictaba Homologs from Arabidopsis thaliana Are Involved in Plant Stress Responses. Front. Plant Sci. 8, 2218 (2018)
Sun, Y., Qiao, Z., Muchero, W., Chen, J.G.: Lectin Receptor-Like Kinases: The Sensor and Mediator at the Plant Cell Surface. Front. Plant Sci. 11, 596301 (2020)
Sahid, S., Roy, C., Paul, S., Datta, R.: Rice lectin protein r40c1 imparts drought tolerance by modulating S-adenosylmethionine synthase 2, stress-associated protein 8 and chromatin-associated proteins. J. Exp. Bot. 71, 7331–7346 (2020)
Hashim, O.H., Jayapalan, J.J., Lee, C.S.: Lectins: an effective tool for screening of potential cancer biomarkers. Peer J. 5, e3784 (2017)
Zhang, L., Luo, S., Zhang, B.: The use of lectin microarray for assessing glycosylation of therapeutic proteins. MAbs 8, 524–535 (2016)
Damodaran, D., Jeyakani, J., Chauhan, A., Kumar, N., Chandra, N.R., Surolia, A.: CancerLectinDB: a database of lectins relevant to cancer. Glycoconj J. 25, 191–198 (2008)
Purohit, S., Li, T., Guan, W., Song, X., Song, J., Tian, Y., Li, L., Sharma, A., Dun, B., Mysona, D., Ghamande, S., Rungruang, B., Cummings, R.D., Wang, P.G., She, J.X.: Multiplex glycan bead array for high throughput and high content analyses of glycan binding proteins. Nat. Commun. 9, 258 (2018)
Haab, B.B., Klamer, Z.: Advances in Tools to Determine the Glycan-Binding Specificities of Lectins and Antibodies. Mol Cell Proteomics. 19, 224–232 (2020)
Dang, K., Zhang, W., Jiang, S., Lin, X., Qian, A.: Application of Lectin Microarrays for Biomarker Discovery. ChemistryOpen. 9, 285–300 (2020)
Poiroux, G., Barre, A., Van Damme, E.J.M., Benoist, H., Rougé, P.: Plant lectins targeting O-glycans at the cell surface as tools for cancer diagnosis, prognosis and therapy. Int. J. Mol. Sci. 18, 1232 (2017)
Benoist, H., Culerrier, R., Poiroux, G., Ségui, B., Jauneau, A., Van Damme, E.J.M., Peumans, W.J., Barre, A., Rougé. P.: Two structurally identical mannose-specific jacalin-related lectins display different effects on human T lymphocyte activation and cell death. J. Leukocyte Biol. 86, 103–114 (2009)
Poiroux, G., Barre, A., Simplicien, M., Pelofy, S., Ségui, B., Van Damme, E.J.M., Rougé, P., Benoist, H.: Morniga G, a T/Tn-specific lectin, induces leukemic cell death via caspase and DR5 receptor-dependent pathways. Int. J. Mol. Sci. 20, 230 (2019)
Poiroux, G., Pitié, M., Culerrier, R., Lafont, E., Ségui, B., Van Damme, E.J.M., Peumans, W.J., Bernadou, J., Levade, T., Rougé, P., Barre, A., Benoist, H.: Targeting of t/tn antigens with a plant lectin to kill human leukemia cells by photochemotherapy. PLoS One 6, e23315 (2011)
Evangelio, E., Poiroux, G., Culerrier, R., Pratviel, G., Van Damme, E.J.M., Peumans, W., Barre, A., Rougé, P., Benoist, H., Pitié, M.: Comparative study of the phototoxicity of long-wavelength photosensitizers targeted by the Morniga G lectin. Bioconj. Chem. 22, 1337–1344 (2011)
Choi, Y., Park, U., Koo, H.J., Park, J.S., Lee, D.H., Kim, K., Choi, J.: Exosome-mediated diagnosis of pancreatic cancer using lectin-conjugated nanoparticles bound to selective glycans. Biosens Bioelectron. 177, 112980 (2021)
Bloise, N., Okkeh, M., Restivo, E., Della Pina, C., Visai, L.: Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis. Nanomaterials 11, 289 (2021)
Martinez, Z.M., De Schutter, K., Van Damme, E.J.M., Vogel, E., Wynant, N., Vanden Broeck, J., Christiaens, O., Smagghe, G.: Accelerated delivery of dsRNA in lepidopteran midgut cells by a GNA lectin-dsRBD fusion protein. Pest. Biochem. Physiol. 175, 104853 (2021)
Balzarini, J.: Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir. Chem. Chemother. 18, 1–11 (2007)
Mitchell, C.A., Ramessar, K., O’Keefe, B.R.: Antiviral lectins: Selective inhibitors of viral entry. Antiviral Res. 142, 37–54 (2017)
Balzarini, J., Schols, D., Neyts, J., Van Damme, E., Peumans, W., De Clercq, E.: alfa-(1–3)- and alfa-(1–6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob. Agents Chemother. 35, 410–416 (1991)
Balzarini, J., Neyts, J., Schols, D., Hosoya, M., Van Damme, E., Peumans, W., De Clercq, E.: The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-Acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 18, 191–207 (1992)
Balzarini, J., Van Laethem, K., Hatse, S., Vermeire, K., De Clercq, E., Peumans, W., Van Damme, E., Vandamme, A., Böhlmstedt, A., Schols, D.: Profile of resistance of human immunodeficiency virus to mannose-specific lectins. J. Virology 78, 10617–10627 (2004)
Balzarini, J., Hatse, S., Vermeire, K., Princen, K., De Clercq, E., Egberink, H., Peumans, W., Van Damme, E., Schols, D.: Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 48, 3858–3870 (2004)
Balzarini, J., Van Laethem, K., Hatse, S., Froeyen, M., Van Damme, E., Bolmstedt, A., Peumans, W., De Clercq, E., Schols, D.: Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol. Pharmacol. 67, 1556–1565 (2005)
Balzarini, J., Van Laethem, K., Hatse, S., Froeyen, M., Peumans, W., Van Damme, E., Schols, D.: Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: A new therapeutic concept to hit the Achilles heel of HIV. J. Biol. Chem. 280, 41005–41014 (2005)
Keyaerts, E., Vijgen, L., Pannecouque, C., Van Damme, E., Peumans, W., Egberink, H., Balzarini, J., Van Ranst, M.: Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 75, 179–187 (2007)
Bertaux, C., Daelemans, D., Meertens, L., Cormier, E.G., Reinus, J.F., Peumans, W.J., Van Damme, E.J.M., Igarashi, Y., Oki, T., Schols, D., Dragic, T., Balzarini, J.: Entry of hepatitis C Virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology 366, 40–50 (2007)
Vanderlinden, E., Van Van Winkel, N., Damme, E.J.M., Naesens, L., Persoons, L., Schols, D.: In vitro characterization of the carbohydrate-binding agents HHA, GNA and UDA as inhibitors of influenza A and B virus replication. Antimicrob. Agents Chemother. 65, e01732-e1820 (2021)
André, S., Kaltner, H., Manning, J.C., Murphy, P.V., Gabius, H.J.: Lectins: getting familiar with translators of the sugar code. Molecules 20, 1788–1823 (2015)
Habermann, F.A., Kaltner, H., Higuero, A.M., Garca Caballero, G., Ludwig, A.K., Manning, J.C., Abad-Rodrguez, J., Gabius, H.J.: What Cyto- and Histochemistry Can Do to Crack the Sugar Code. Acta Histochem. Cytochem. 54, 31–48 (2021)
Flynn, R.A., Pedram, K., Malaker, S.A., Batista, P.J., Smith, B.A.H., Johnson, A.G., George, B.M., Majzoub, K., Villalta, P.W., Carette, J.E., Bertozzi, C.R.: Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 184, 3109-3124.e22 (2021)
Schouppe, D., Ghesquière, B., Menschaert, G., De Vos, W.H., Bourque, S., Trooskens, G., Proost, P., Gevaert, K., Van Damme, E.J.M.: Interaction of the tobacco lectin with histone proteins. Plant Physiol. 155, 1091–1102 (2011)
Delporte, A., De Zaeytijd, J., De Storme, N., Azmi, A., Geelen, D., Smagghe, G., Guisez, Y., Van Damme, E.J.M.: Cell cycle–dependent O-GlcNAc modification of tobacco histones and their interaction with the tobacco lectin. Plant Physiol. Biochem. 83, 151–158 (2014)
Delporte, A., De Vos, W.H., Van Damme, E.J.M.: In vivo interaction between the tobacco lectin and the core histone proteins. J. Plant Physiol. 171, 1149–1156 (2014)
De Coninck, T., Gistelinck, K., Janse van Rensburg, H.C., Van den Ende, W., Van Damme, E.J.M.: Sweet modifications modulate plant development. Biomolecules. 11, 756 (2021)
Goddard, T.D., Huang, C.C., Meng, E.C., Pettersen, E.F., Couch, G.S., Morris, J.H., Ferrin, T.E.: UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018)
Acknowledgements
This research was funded by many grants from the Fund for Scientific Research – Flanders, Catholic University of Leuven, Ghent University and the Francqui Research Foundation.
I am grateful to all students, postdocs, and collaborators who have contributed to the lectin project over the years. My special thanks are due to Prof. W. Peumans, Ph.D. (Leuven, Belgium), Prof. I. Goldstein, Ph.D. (Ann Arbor, USA), Prof. P. Rougé, Ph.D. (Toulouse, France), Prof G. Smagghe, Ph.D. (Gent, Belgium), Prof D. Schols, Ph.D. and Prof J. Balzarini, Ph.D. (Leuven, Belgium) for their help, support and long-lasting collaborations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van Damme, E.J.M. 35 years in plant lectin research: a journey from basic science to applications in agriculture and medicine. Glycoconj J 39, 83–97 (2022). https://doi.org/10.1007/s10719-021-10015-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-021-10015-x