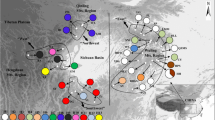
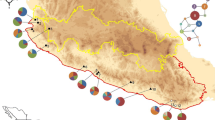
Abstract
Climate changes during glacial periods have had significant effects on the current distribution of plant species. Palaeontologial data suggest that modern cycads originated in southwest China. Cycas debaoensis (Cycadaceae) is an endangered species restricted to a small area of southwest China. This species has been classified into two types: sand and karst, according to the soil matrix they grow on. To determine the locations of its glacial refugia and its genetic structure, we examined chloroplast sequence variation of the atpB-rbcL and psbA-trnH intergenic spacers. Four chloroplast DNA haplotypes were obtained from 120 individuals collected from 11 populations covering the entire extant distribution range of the species. Significant population subdivision was detected (G ST = 0.684 and F ST = 0.74160), suggesting low levels of gene flow between regions and populations. There was marked haplotype differentiation between populations in the sand and karst regions, with only one haplotype being present in both. The molecular phylogenetic data, together with the geographic distribution of the haplotypes, suggest that C. debaoensis experienced range contraction during glacial periods, and that the current populations are still confined to the areas of the original refugia. These results implied that isolated refugia might have maintained in both sand and karst regions during the last glacial maximum and even earlier glaciations. The low within-population diversity of C. debaoensis suggested that there were strong bottleneck events or founder effects within each separate region during the Quaternary climatic oscillations. These findings are important for the conservation of this endangered species.




Similar content being viewed by others
References
Amos W, Harwood J (1998) Factors affecting levels of genetic diversity in natural populations. Philos Trans R Soc Lond B Biol Sci 353:177–186
Artyukova EV, Kozyrenko MM, Gorovoy PG, Zhuravlev YN (2009) Plastid DNA variation in highly fragmented populations of Microbiota decussata Kom. (Cupressaceae), an endemic to Sikhote Alin mountains. Genetica 137:201–212
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Barrett SCH, Kohn JR (1991) Genetic and evolutionary consequences of small population size in plants: implications for conservation. Oxford University Press, New York
Burns EL, Eldridge MDB, Crayn DM, Houlden BA (2007) Low phylogeographic structure in a wide spread endangered Australian frog Litoria aurea (Anura: Hylidae). Conserv Genet 8:17–32
Cabrera-Toledo D, González-Astorga J, Vovides AP (2008) Heterozygote excess in ancient populations of the critically endangered Dioon caputoi (Zamiaceae, Cycadales) from central Mexico. Bot J Linn Soc 158:436–447
Cabrera-Toledo D, González-Astorga J, Nicolalde-Morejón F, Vergara-Silva F, Vovides AP (2010) Allozyme diversity levels in two congeneric Dioon spp. (Zamiaceae, Cycadales) with contrasting rarities. Plant Syst Evol 290:115–125
Cafasso D, Cozzolino S, Caputo P, Luca PD (2001) Maternal inheritance of plastids in Encephalartos Lehm (Zamiaceae, Cycadales). Genome 44:239–241
Cai YL, Wang XH, Song YC (1999) An ecoanatomical study on leaves of Cyclobalanopsis glauca populations in the eastern subtropical zone, China. Acta Ecologica Sinica 19:844–849
Chiang YC, Hung KH, Moore SJ, Ge XJ, Hung S, Hsu TW, Schaal BA, Chiang TY (2009) Paraphyly of organelle DNAs in Cycas Sect. Asiorientales due to ancient ancestral polymorphisms. BMC Evol Biol 9:161
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochem Bull 19:11–15
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Ellstrand NC, Ornduff R, Clegg JM (1990) Genetic structure of the Australian cycad, Macrozamia communis (Zamiaceae). Am J Bot 77:677–681
Erdei B, Akgün F, Barone Lumaga MR (2010) Pseudodioon akyoli gen. et sp. nov., an extinct member of Cycadales from the Turkish Miocene. Plant Syst Evol 285:33–49
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Franco AM, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R, Huntley B, Thomas CD (2006) Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Global Change Biol 12:1545–1553
Frankel O, Soulé M (1981) Conservation and evolution. Cambridge University Press, Cambridge
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Fu LG, Jin JM (1992) China plant red data book: rare and endangered plants, I. Science Press, Beijing
Gao ZF, Thomas BA (1989) A review of fossil cycad megasporophylls, with new evidence of Crossozamia pomel and its associated leaves from the lower Permian of Taiyuan, China. Rev Palaeobot Palynol 60:205–223
González-Astorga J, Vovides AP, Ferrer MM, Iglesias C (2003) Population genetics of Dioon edule Lindl. (Zamiaceae, Cycadales): biogeographical and evolutionary implications. Biol J Linn Soc 80:457–467
González-Astorga J, Vovides A, Cruz-Angon A, Octavio-Aguilar P, Iglesias C (2005) Allozyme variation in the three extant populations of the narrowly endemic Cycad Dioon angustifolium Miq. (Zamiaceae) from North-eastern Mexico. Ann Bot 95:999
GonzáLez-Astorga J, Vergara-Silva F, Vovides AP, Nicolalde-Morejón F, Cabrera-Toledo DAN, Pérez-Farrera MA (2008a) Diversity and genetic structure of three species of Dioon Lindl. (Zamiaceae, Cycadales) from the Pacific seaboard of Mexico. Biol J Linn Soc 94:765–776
González-Astorga J, Vovides AP, Cabrera-Toledo D, Nicolalde-Morejón F (2008b) Diversity and genetic structure of the endangered cycad Dioon sonorense (Zamiaceae) from Sonora, Mexico: evolutionary and conservation implications. Biochem Syst Ecol 36:891–899
Graur D, Li WH (2000) Fundamentals of molecular evolution. Sinauer Associates, Sunderland
Guan ZT, Zhou L (1996) Cycads of China. Sichuan Science and Technology Press, Chengdu
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. New Forest 6:95–124
Hendricks JG (1987) The Gondwanan Cycas. Encephalartos 10:24–25
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Hewitt GM (1996) Some genetic consequences of ice ages, and their role, in divergence and speciation. Biol J Linn Soc 58:247–276
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond Ser B Biol Sci 359:183–195
Hill KD, Stevenson DW, Osborne R (2004) The world list of cycads. Bot Rev 70:274–298
Hu YF, Chen ZD, Chen CJ, Geng BY (1999) Discoveries of some fossils of cycad reproductive organs from China and their significance to the origin of cycads. In: Biology and conservation of cycads-proceedings of the fourth international conference on cycad biology. International Academic Publishers, Beijing, pp. 43–48
Ikeda H, Senni K, Fujii N, Setoguchi H (2008) Consistent geographic structure among multiple nuclear sequences and cpDNA polymorphisms of Cardamine nipponica Franch. et Savat. (Brassicaceae). Mol Ecol 17:3178–3188
Jian SJ, Zhong Y, Liu N, Gao ZZ, Wei Q, Xie ZH, Ren H (2006) Genetic variation in the endangered endemic species Cycas fairylakea (Cycadaceae) in China and implications for conservation. Biodivers Conserv 15:1681–1694
Keppel G, Lee SW, Hodgskiss PD (2002) Evidence for long isolation among populations of a Pacific cycad: genetic diversity and differentiation in Cycas seemannii A. Br. (Cycadaceae). J Hered 93:133–139
Kyoda S, Setoguchi H (2010) Phylogeography of Cycas revoluta Thunb. (Cycadaceae) on the Ryukyu islands: very low genetic diversity and geographical structure. Plant Syst Evol 288:177–189
Li PJ (1982) Early Cretaceous plants from the Tuoni formation of eastern Xizang. People’s Press of Sichuan, Chengdu
Li P, Cao Z, Wu S (1976) Mesozoic plants of Yunnan, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences edn. Science Press, Beijing
Liao PC, Havanond S, Huang S (2007) Phylogeography of Ceriops tagal (Rhizophoraceae) in Southeast Asia: the land barrier of the Malay Peninsula has caused population differentiation between the Indian Ocean and South China Sea. Conserv Genet 8:89–98
Liepelt S, Bialozyt R, Ziegenhagen B (2002) Wind-dispersed pollen mediates postglacial gene flow among refugia. Proc Natl Acad Sci USA 99:14590–14594
Lin TP, Sun YC, Lo HC, Cheng YP (2000) Low genetic diversity of Cycas taitungensis (Cycadaceae), an endemic species in Taiwan, revealed by allozyme analysis. Taiwan J For Sci 14:35–42
Lynch M (1996) A quantitative-genetic perspective on conservation issues. Chapman and Hall, New York
Ma XY, Jian SG, Wu M, Liu N (2003) The population characters and conservation of Cycas debaoensis Y.C. Zhong et. C. J. Chen. Guihaia 23:123–126
Maxted N, Hawkes JG, Ford-Lloyd BV (1997) Selection of target taxa. Chapman & Hall, London
Moritz C (1994) Defining “Evolutionarily significant units” for conservation. Trends Ecol Evol 9:373–374
Naciri Y, Gaudeul M (2007) Phylogeography of the endangered Eryngium alpinum L. (Apiaceae) in the European Alps. Mol Ecol 16:2721–2733
Norstog KJ, Nicholls TJ (1997) The biology of the cycads. Cornell University Press, Ithaca
Opgenoorth L, Vendramin GG, Mao K, Miehe G, Miehe S, Liepelt S, Liu J, Ziegenhagen B (2010) Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the last glacial maximum. New Phytol 185:332–342
Petit RJ, Aguinagalde I, de Beaulieu JL, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565
Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG (2005) Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol 14:689–701
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Posada D, Crandall KA (1998) MODEL TEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Provan J, Powell W, Hollingsworth PM (2001) Chloroplast microsatellites: new tools for studies in plant ecology and evolution. Trends Ecol Evol 16:142–147
Rambaut A, Drummond A (2004) Tracer: MCMC trace analysis tool. University of Oxford, Oxford
Reboud X, Zeyl C (1994) Organelle inheritance in plants. Heredity 72:132–140
Rendell S, Ennos RA (2003) Chloroplast DNA diversity of the dioecious European tree Ilex aquifolium L. (English holly). Mol Ecol 12:2681–2688
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497
Schaal BA, Da-hayworth KMO, Rauscher JT, Smith WA (1998) Phylogeographic studies in plants: problems and prospects. Mol Ecol 7:465–474
Taberlet P, Fumagalli L, Wust-Saucy AG, Cosson JF (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–589
Templeton AR, Sing CF (1993) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping. IV. Nested analyses with cladogram uncertainty and recombination. Genetics 134:659–669
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Van Valen L (1965) Morphological variation and width of ecological niche. Am Nat 99:377–390
Walter R, Epperson BK (2005) Geographic pattern of genetic diversity in Pinus resinosa: contact zone between descendants of glacial refugia. Am J Bot 92:92–100
Walters TW, Decker-Walters DS (1991) Patterns of allozyme diversity in the West Indies cycad Zamia pumila (Zamiaceae). Am J Bot 78:436–445
Wang CH (2007) Population biosystematic and conservation biology of Cycas debaoensis. Guangxi Normal University, Guilin
Wang X, Li N, Wang YD, Zheng SL (2009) The discovery of whole-plant fossil cycad from the upper Triassic in western Liaoning and its significance. Chin Sci Bull 54:3116–3119
Wang H, Qiong L, Sun K, Lu F, Wang YG, Song ZP, Wu QH, Chen JK, Zhang WJ (2010) Phylogeographic structure of Hippophae tibetana (Elaeagnaceae) highlights the highest microrefugia and the rapid uplift of the Qinghai-Tibetan Plateau. Mol Ecol 19:2964–2979
Wu GY (1990) Main petrochemistry features of yakou gneiss. Acta Petrol Sin 6:82–91
Xiao LQ, Ge XJ, Gong X, Hao G, Zheng SX (2004) ISSR variation in the endemic and endangered plant Cycas guizhouensis (Cycadaceae). Ann Bot 94:133–138
Xie JG, Jian SG, Liu N (2005) Genetic variation in the endemic plant Cycas debaoensis on the basis of ISSR analysis. Aust J Bot 53:141–146
Yang SL, Meerow AW (1996) The Cycas pectinata (Cycadaceae) complex: genetic structure and gene flow. Int J Plant Sci 157:468–483
Zhai MG, Yang RY (1986) Early Precambrian gneiss basement in the Panxi area, southwest China. Acta Petrol Sin 2:22–37
Zhang JW, Yao JX, Chen JR, Li CS (2010) A new species of Leptocycas (Zamiaceae) from the upper Triassic sediments of Liaoning province, China. J Syst Evol 48:286–301
Zhong YC, Chen CJ (1997) Cycas debaoensis Y.C. Zhong et C.J. Chen—a new cycad from China. Acta Phytotaxon Sin 35:571
Acknowledgments
We wish to thank Wang Chao-Hong for her help in collection of material and thank Liao Pei-Chun and Chiang Yu-Chung for data analysis. We are grateful to He Wang-Long for help with the haplotype plotting. We would also like to thank Chen Jia-Rui for valuable suggestions. This study was supported by grants from the National Natural Science Foundation of China (30670210) and the National Basic Research Program of China (973 Program: 2007CB411600).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qing-Qing Zhan and Jin-Feng Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhan, QQ., Wang, JF., Gong, X. et al. Patterns of chloroplast DNA variation in Cycas debaoensis (Cycadaceae): conservation implications. Conserv Genet 12, 959–970 (2011). https://doi.org/10.1007/s10592-011-0198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-011-0198-9