Abstract
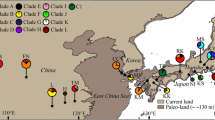
The dove tree, Davidia involucrata Baill. (Davidiaceae), is a relict species endemic to China. Human activities and the strict requirements for seedling recruitments have led to the decline in number of individuals in extant natural populations of this species. In order to provide information for the design of conservation management strategies for D. involucrata, we investigated the phylogeographical pattern of this relict tree. Our sampling included 256 individuals from 32 natural populations of D. involucrata in China and we sequenced six chloroplast DNA (cpDNA) non-coding regions. We distinguished a total of 13 different cpDNA haplotypes. From the cpDNA variation in D. involucrata, we found a very high level of regional differentiation (F ST = 0.812) and a strong phylogeographical pattern (N ST = 0.996 > G ST = 0.981, P < 0.05). Phylogenetic analysis reveals three main cpDNA haplotype lineages and four population groups. The split between these geographical groups can be dated back from the late Pliocene to early Pleistocene. Non-overlapping distribution of chloroplast haplotypes and high genetic differentiation among four distinct geographical groups suggest that D. involucrata probably survives in four separate glacial refugia. Our findings have an important implication for conservation of its genetic diversity. The deduced glacial survival areas for D. involucrata should be recognized as four “evolutionary significant units” and be considered as separate targets in conserving its genetic diversity.


Similar content being viewed by others
References
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Axelrod DI, Al-Shehba I, Raven PH (1996) History of the modern flora of China. In: Zhang AL, Wu SG (eds) Floristic characteristics and diversity of East Asian plants. Springer, New York, pp 43–55
Bai WN, Liao WJ, Zhang DY (2010) Nuclear and chloroplast DNA phylogeography reveal two refuge areas with asymmetrical gene flow in a temperate walnut tree from East Asia. New Phytol 188:892–901
Budantsev LY (2006) The western Kamchatka early Paleogene flora. Trans Bot Inst Russian Acad Sci 22:1–160
Byrne M (2007) Phylogeography provides an evolutionary context for the conservation of a diverse and ancient flora. Aust J Bot 55:316–325
Chiang TY, Schaal BA (1999) Phylogeography of ten North American Hylocomium splendens based on nrDNA ITS sequences. Mol Ecol 8:1037–1042
Chou YW, Thomas PI, Ge XJ, LePage BA, Wang CN (2011) Refugia and phylogeography of Taiwania in East Asia. J Biogeogr 38:1992–2005
Clement M, Posada D, Crandall K (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
Crandall KA, Templeton AR (1993) Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 134:959–969
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214–221
Du YJ (2012) Studies on population genetics and phylogeography of Davidia involucrata (Davidiaceae). PH.D. Dissertation, Zhejiang University, Hangzhou (in Chinese with English abstract)
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11:2571–2581
Eiserhardt WL, Svenning JC, Kissling WD, Balslev H (2011) Geographical ecology of the palms (Arecaceae): determinants of diversity and distributions across spatial scales. Ann Bot 108:1391–1416
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–259
Escuderoa A, Iriondob JM, Torres ME (2003) Spatial analysis of genetic diversity as a tool for plant conservation. Biol Conserv 113:351–365
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2006) ARLEQUIN version 3.1: a software for population genetic data analysis. Computational and molecular population genetics laboratory, Institute of Zoology, University of Berne, Switzerland
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitch-hiking, and background selection. Genetics 147:915–925
Fu DZ, Endress PK (2001) Cercidiphyllaceae. In: Wu ZY, Raven PH (eds) Flora of China, vol. 6. Science Press, Beijing; Missouri Botanical Garden Press, St. Louis, MO, p 126
Fu LK, Jin JM (1992) China plant red data book. Science Press, Beijing
Gao LM, Moller M, Zhang XM, Hollingsworth ML, Liu J, Mill RR, Gibby M, Li DZ (2007) High variation and strong phylogeographic pattern among cpDNA haplotypes in Taxus wallichiana (Taxaceae) in China and North Vietnam. Mol Ecol 16:4684–4698
Ge XJ, Chiang YC, Chou CH, Chiang TY (2002) Nested clade analysis of Dunnia sinensis, a monotypic genus of the Rubiaceae, from China based on organelle DNA sequences. Conserv Genet 3:351–362
Gladenkov YB, Sinel’nikova VN, Chelebaeva AI, Shantser AE (2005) Biosphere–Ecosystem–Biota during the Earth’s past. Ecosystems in the Cenozoic of the Northern Pacific. Eocene–Oligocene of western Kamchatka and adjacent regions (Contribution to Academician V.V. Menner’s 100th Birthday)
Gong W, Chen C, Dobes C, Fu CX, Koch MA (2008) Phylogeography of a living fossil, Pleistocene glaciations forced Ginkgo biloba L. (Ginkgoaceae) into two refuge areas in China with limited subsequent postglacial expansion. Mol Phylogenet Evol 48:1094–1105
Guan BC, Fu CX, Qiu YX, Zhou SL, Comes HP (2010) Genetic structure and breeding system of a rare understory herb, Dysosma versipellis (Berberidaceae), from temperate deciduous forests in China. Am J Bot 97:111–122
Harpending HC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Harrison SP, Yu G, Takahara H, Prentice IC (2001) Palaeovegetation: diversity of temperate plants in East Asia. Nature 413:129–130
He JS, Lin H, Chen WL (1995) The current status of endemic and endangered species Davidia involucrata and the preserving strategies. Chin Biodivers 3:213–221
Heuertz M, Fineschi S, Anzidei M, Pastorelli R, Salvini D, Paule L, Rascaria-Lacoste N, Hardy OJ, Vekemans X, Vendramin GG (2004) Chloroplast DNA variation and postglacial recolonization of common ash (Fraxinus excelsior L.) in Europe. Mol Ecol 13:3437–3452
Huang S, Chiang YC, Schaal BA, Chou CH, Chiang TY (2001) Organelle DNA phylogeography of Cycas taitungensis, a relict species in Taiwan. Mol Ecol 10:2669–2681
Kokawa S (1965) Fossil endocarp of Davidia in Japan. J Biol, Osaka City University 16: 45–51
Lei M, Wang Q, Wu ZJ, Lopez-Pujol J, Li DZ, Zhang ZY (2012) Molecular phylogeography of Fagus engleriana (Fagaceae) in subtropical China: limited admixture among multiple refugia. Tree Genet Genomes 8:1203–1212
Li XW, Li J (1993) A preliminary floristic study on the seed plants from the region of Hengduan Mountain. Acta Bot Yunnan 15:217–231 (in Chinese with English abstract)
Li JQ, Zhang MH, Huang HW, Cai Q (2000) On the allozymic loci variation of Davidia involucrata. J Wuhan Bot Res 18:247–249 (in Chinese with English abstract)
Li XP, He ZQ, Chen FJ, Liang HW, Li FL (2006) RAPD analysis for the genetic diversity of four populations of Davidia involucrata Baill. in Shennongjia area, Hubei Province. J Beijing For Univ 28:66–70 (in Chinese with English abstract)
Li AX, Yi S, Qiu YX, Guo JT, Comes HP, Fu CX (2008) Phylogeography of two East Asian species in Croomia (Stemonaceae) inferred from chloroplast DNA and ISSR fingerprinting variation. Mol Phylogenet Evol 49:702–714
Li XP, Li ZL, He CL, Zhu WY, Gao SP (2012a) Genetic diversity of the endangered Davidia involucrata by AFLP analysis. Acta Hortic Sin 39:992–998 (in Chinese with English abstract)
Li XP, Zheng X, Zhu WY, He CL, Gao SP (2012b) Genetic diversity and genetic structure analysis of the endangered Davidia involucrata Baill. by ISSR. Guangdong Agric Sci 6:121–123 (in Chinese with English abstract)
Luo SJ, He YH, Ning GG, Zhang JQ, Ma GY, Bao MZ (2011) Genetic diversity and genetic structure of different populations of the endangered species Davidia involucrata in China detected by inter-simple sequence repeat analysis. Trees 25:1063–1071
Mancheste SR, Chen ZD, Lu AM, Uemura K (2009) Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J Syst Evol 47:1–42
Manchester SR (2002) Leaves and fruits of Davidia (Cornales) from the Paleocene of North America. Syst Bot 27:368–382
Moritz C (1994) Defining significant units for conservation. Trends Ecol Evol 9:373–375
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Pavlyutkin BI (2009) Leaf and fruit remains of Davidia (Cornales) from the Nezhino flora (Miocene of Primorye). Paleontol J 43:339–344
Peng YL, Hu YQ, Sun H (2003) Allozyme analysis of Davidia involucrata var. vilmoriniana and its biogeography significance. Acta Bot Yunnan 25:55–62 (in Chinese with English abstract)
Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG (2005) Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol 14:689–701
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered allels. Genetics 144:1237–1245
Qi XS, Chen C, Comes HP, Sakaguchi S, Liu YH, Tanaka N, Sakio H, Qiu YX (2012) Molecular data and ecological niche modeling reveal a highly dynamic evolutionary history of the East Asian Tertiary relict Cercidiphyllum (Cercidiphyllaceae). New Phytol 196:617–630
Qiu YX, Guan BC, Fu CX, Comes HP (2009a) Did glacials and/or interglacials promote allopatric incipient speciation in East Asian temperate plants? Phylogeographic and coalescent analyses on refugial isolation and divergence in Dysosma versipellis. Mol Phylogenet Evol 51:281–293
Qiu YX, Qi XS, Jin XF, Tao XY, Fu CX, Naiki A, Comes HP (2009b) Population genetic structure, phylogeography, and demographic history of Platycrater arguta (Hydrangeaceae) endemic to East China and South Japan, inferred from chloroplast DNA sequence variation. Taxon 58:1226–1241
Qiu YX, Sun Y, Zong M, Zhang XP, Lee J, Murata J, Fu CX, Comes HP (2009c) Molecular phylogeography of East Asian Kirengeshoma in relation to quaternary climate change and land-bridge configurations. New Phytol 183:480–495
Qiu YX, Fu CX, Comes HP (2011) Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol Phylogenet Evol 59:225–244
Rambaut A, Drummond AJ (2007) Tracer v1.4. Available from http://beast.bio.ed.ac.uk/Tracer
Ray N, Adams JM (2001) A GIS-based vegetation map of the world at the last glacial maximum (25,000–15,000 BP). Internet archaeology, 11. Available from: http://www.ncdc.noaa.gov/paleo/pubs/ray2001/ray_adams_2001.pdf/
Ren G, Beug HJ (2002) Mapping holocene pollen data and vegetation of northern China. Quat Sci Rev 21:1395–1422
Rivera-Ocasio E, Aide TM, McMillan WO (2006) The influence of spatial scale on the genetic structure of a widespread tropical wetland tree, Pterocarpus officinalis (Fabaceae). Conserv Genet 7:251–266
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Saeki I, Murakami N (2009) Chloroplast DNA phylogeography of the endangered Japanese red maple (Acer pycnanthum): the spatial configuration of wetlands shapes genetic diversity. Divers Distrib 15:917–927
Schneider S, Excoffier L (1999) Estimation of demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152:1079–1089
Song CW, Bao MZ (2006) Genetic diversity of RAPD mark for natural Davidia involucrata populations. Front China 1:95–99
Sun JF, Gong YB, Renner SS, Huang SQ (2008) Multifunctional bracts in the dove tree Davidia involucrata (Nyssaceae: Cornales): rain protection and pollinator attraction. Am Nat 171:119–124
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tian B, Liu RR, Wang LY, Qiu Q, Chen KM, Liu JQ (2009) Phylogeographic analyses suggests that a deciduous species (Ostryopsis davidiana Decne., Betulaceae) survived in northern China during the Last Glacial Maximum. J Biogeogr 36:2148–2155
Tsukagosmh I, Ono Y, Hashimot T (1997) Fossil endocarp of Davidia from the early Pleistocene sediments of the Tokai group in Gifu prefecture, central Japan. Bull Osaka Mus Nat Hist 51:13–23
Wang HS (1992) Floristic geography. Science Press, Beijing (in Chinese)
Wang HW, Ge S (2006) Phylogeography of the endangered Cathaya argyrophylla (Pinaceae) inferred from sequence variation of mitochondrial and nuclear DNA. Mol Ecol 15:4109–4122
Wang J, Gao PX, Kang M, Lowe AJ, Huang HW (2009) Refugia within refugia: the case study of a canopy tree Eurycorymbus cavaleriei in subtropical China. J Biogeogr 36:2156–2164
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058
Wu ZY, Wu SG (1996) A proposal for a new floristic kingdom (realm)—the E. Asiatic kingdom, its delimitation and characteristics. In: Zhang AL, Wu SG (eds) Proceedings of the first international symposium on floristic characteristics and diversity of East Asian plants. China Higher Education Press/Springer-Verlag, Beijing, China/Berlin, Heidelberg, Germany, pp 3–42
Wu G, Xiao H, Li J, Ma KM (2000) Relationship between human activities and survival of rare and endangered species Davidia involucrata. Chin J Appl Ecol 11:493–496 (in Chinese with English abstract)
Xiang QY, Thomas DT, Xiang QP (2011) Resolving and dating the phylogeny of Cornales—effects of taxon sampling, data partitions, and fossil calibrations. Mol Phylogenet Evol 59:123–138
Xu GB, Yu YT, Shen XB (2007) Studies of genetic diversity of dove trees (Davidia involucrate) in west Hunan and Hubei by RAPD. J Central South Univ For Technol 27:5–9 (in Chinese with English abstract)
Ying TS (2001) Species diversity and distribution pattern of seed plants in China. Biodivers Sci 9:393–398 (in Chinese with English abstract)
Ying TS, Zhang YL, Boufford DE (1993) The endemic genera of seed plants of China. Science Press, Beijing (in Chinese)
Yu YF (1999) A milestone of wild plant conservation in China. Plants 5:3–11 (in Chinese)
Zhan YY, Liu YH, Xiong WJ (2010) Current situation and prospect of endangered reason of Davidia involucrate Baill. Hubei For Sci Technol 161:41–56 (in Chinese with English abstract)
Zhang YM (2012) The research on genetic diversity and molecular phylogenetic population of Davidia involucrata. Master Dissertation, Central South University of Forestry Technology, Changsha (in Chinese with English abstract)
Zhang RZ, Zheng D, Yang QY, Liu YH (1997) Physical geography of Hengduan Mountains. Science Press, Beijing (in Chinese)
Acknowledgments
The authors thank Ling-Yun Chen, Xiao-Li Yue, and Dan Yang for their help in fieldwork, Zhi-Yuan Du and Dun Wang for their assistance in the laboratory, and Hans Peter Comes for his useful comments and suggestions on an earlier draft of the manuscript. This study was supported by grants from One Hundred Person Project of the Chinese Academy of Sciences granted to WQF (KSCX2-YW-Z-0805), the strategic pilot science and technology projects of the Chinese Academy of Sciences (Grant No. XDAO5090305), and the National Natural Science Foundation of China (No. 31270278).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin-Ming Chen and Shu-Ying Zhao contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10592_2014_683_MOESM2_ESM.docx
Supplementary material 2 (DOCX 18 kb) Table S2 Chloroplast DNA sequence polymorphism detected in six non-coding regions of Davidia involucrata identifying 13 haplotypes (H1-13). Dot (.) shows a site with the same nucleotide variant to that of the sequence of haplotype H1, “-” indicates alignment gap
10592_2014_683_MOESM3_ESM.doc
Supplementary material 3 (DOC 53 kb) Table S3 GenBank accession numbers for each of the six non-coding regions of each chloroplast haplotype (H1-13) identified in Davidia involucrata
10592_2014_683_MOESM4_ESM.doc
Supplementary material 4 (DOC 43 kb) Fig. S1 Distribution of the number of pairwise nucleotide differences for six non-coding regions sequence data in Davidia involucrata. a The whole species; b The WLS mountain region (HPG I); c The western region of Sichuan Basin (HPG II + III). The dash line represents observed value whereas the solid line shows expected values under a sudden (stepwise) population expansion
Rights and permissions
About this article
Cite this article
Chen, JM., Zhao, SY., Liao, YY. et al. Chloroplast DNA phylogeographic analysis reveals significant spatial genetic structure of the relictual tree Davidia involucrata (Davidiaceae). Conserv Genet 16, 583–593 (2015). https://doi.org/10.1007/s10592-014-0683-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-014-0683-z