Abstract
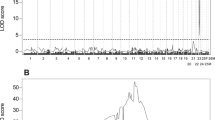
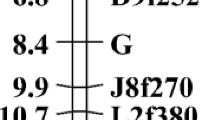
We previously reported a body color deformity in juvenile red sea bream, which shows transparency in the juvenile stage because of delayed chromatophore development compared with normal individuals, and this finding suggested a genetic cause based on parentage assessments. To conduct marker-assisted selection to eliminate broodstock inheriting the causative gene, developing DNA markers associated with the phenotype was needed. We first conducted SNP mining based on AFLP analysis using bulked-DNA from normal and transparent individuals. One SNP was identified from a transparent-specific AFLP fragment, which significantly associated with transparent individuals. Two alleles (A/G) were observed in this locus, and the genotype G/G was dominantly observed in the transparent groups (97.1%) collected from several production lots produced from different broodstock populations. A few normal individuals inherited the G/G genotype (5.0%), but the A/A and A/G genotypes were dominantly observed in the normal groups. The homologs region of the SNP was searched using a medaka genome database, and intron 12 of the Nell2a gene (located on chromosome 6 of the medaka genome) was highly matched. We also mapped the red sea bream Nell2a gene on the previously developed linkage maps, and this gene was mapped on a male linkage group, LG4-M. The newly found SNP was useful in eliminating broodstock possessing the causative gene of the body color transparency observed in juvenile stage of red sea bream.



Similar content being viewed by others
References
Agawa Y, Iwaki M, Komiya T, Honryo T, Tamura K, Okada T, Yagishita N, Kobayashi T, Sawada Y (2015) Identification of male sex-linked DNA sequence of the cultured Pacific bluefin tuna Thunnus orientalis. Fish Sci 81:113–121
Chalhoub BA, Thibault S, Laucou V, Rameau C, Höfte H, Cousin R (1997) Silver staining and recovery of AFLP amplification products on large denaturing polyacrylamide gels. BioTechniques 22:216–220
Chatain B (1994) Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119:371–379
Chen SL, Li J, Deng SP, Tian YS, Wang QY, Zhuang ZM, Sha ZX, Xu JY (2007) Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis). Mar Biotechnol 9:273–280
Ezaz MT, Harvey SC, Boonphakdee C, Teale AJ, McAndrew BJ, Penman DJ (2004) Isolation and physical mapping of sex-linked AFLP markers in Nile tilapia (Oreochromis niloticus L.) Mar Biotechnol 6:435–445
Haga Y, Suzuki T, Takeuchi T (2002) Retinoic acid isomers produce malformations in postembryonic development of the Japanese flounder, Paralichthys olivaceus. Zool Sci 19:1105–1112
Harris A, Bieger S, Doyle RW, Wright JM (1991) DNA fingerprinting of tilapia, Oreochromis niloticus, and its application to aquaculture genetics. Aquaculture 92:157–163
Hattori M, Sawada Y, Kurata M, Yamamoto S, Kato K, Kumai H (2004) Oxygen deficiency during somitogenesis causes centrum defects in red sea bream, Pagrus major (Temminck et Schlegel). Aquac Res 35:850–858
Kihara M, Ogata S, Kawano N, Kubota I, Yamaguchi R (2002) Lordosis induction in juvenile red sea bream, Pagrus major, by high swimming activity. Aquaculture 212:149–158
Kitajima C, Watanabe T, Tsukashima Y, Fujita S (1994) Lordotic deformation and abnormal development of swimbladders in some hatchery-bred marine physoclistous fish in Japan. J World Aquacult Soc 25:64–77
Koshimizu E, Strussmann CA, Okamoto N, Fukuda H, Sakamoto T (2010) Construction of a genetic map and development of DNA markers linked to the sex-determining locus in the Patagonian pejerrey (Odontesthes hatcheri). Mar Biotechnol 12:8–13
Kuroda S, Oyasu M, Kawakami M, Kanayama N, Tanizawa K, Saito N, Abe T, Matsuhashi S, Ting K (1999) Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun 265:79–86
Ma H, Chen S, Yang J, Ji X, Chen S, Tian Y, Bi J (2010) Isolation of sex-specific AFLP markers in spotted halibut (Verasper variegatus). Environ Biol Fish 88:9–14
Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K (1995) New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn 203:212–222
Miyashita S, Seoka M (2005) Red Sea bream. In: Kumai H (ed) AquacultureSystem 1. Koseisha-Koseikaku, Tokyo, pp 45–82 (in Japanese)
Murata O, Harada T, Miyashita S, Izumi K, Maeda S, Kato K, Kumai H (1996) Selective breeding for growth in red sea bream. Fish Sci 62:845–849
Oshima N, Suzuki M, Yamaji N, Fujii R (1988) Pigment aggregation is triggered by an increase in free calcium ions within fish chromatophores. Comp Biochem Physiol A Physiol 91:27–32
Qin Y, Liu X, Zhang H, Zhang G, Ximing G (2007) Identification and mapping of amplified fragment length polymorphisms markers linked to shell color in bay scallop, Argopecten irradians irradians (Lamarck, 1819). Mar Biotechnol 9:66–73
Rao HO, Deng JC, Wang WM, Gao ZX (2012) An AFLP-based approach for the identification of sex-linked markers in blunt snout bream, Megalobrama amblycephala (Cyprinidae). Genet Mol Res 11:1027–1031
Santiago-Sotelo P, Ramirez-Prado JH (2012) prfectBLAST: a platform-independent portable front end for the command terminal BLAST+ stand-alone suite. BioTechniques 53:299–300
Sawayama E, Takagi M (2010) Characterization and sibship estimation of body color variants in Japanese flounder Paralichthys olivaceus. Aquac Sci 58:345–350 (in Japanese with English abstract)
Sawayama E, Takagi M (2011) Genetic factors associated with transparency of juvenile red sea bream, Pagrus major. Nippon Suisan Gakkaishi 77:630–638 (in Japanese with English abstract)
Sawayama E, Takagi M (2012) Genetic investigation of artificially raised red sea bream with abnormal vertebrae formation. Nippon Suisan Gakkaishi 78:62–68 (in Japanese with English abstract)
Sawayama E, Takagi M (2016) Genetic diversity and structure of domesticated strains of red sea bream, Pagrus major, inferred from microsatellite DNA markers. Aquac Res 47:379–389
Sawayama E, Kitamura SI, Nakayama K, Ohta K, Ozaki A, Takagi M (2017) Identification of quantitative trait loci for resistance to RSIVD in red sea bream (Pagrus major). Mar Biotechnol 19:601–613
Shikano T (2005) Marker-based estimation of heritability for body color variation in Japanese flounder Paralichthys olivaceus. Aquaculture 249:95–105
Uchino T, Nakamura Y, Sekino M, Kai W, Fujiwara A, Yasuike M, Sugaya T, Fukuda H, Sano M, Sakamoto T (2016) Constructing genetic linkage maps using the whole genome sequence of Pacific bluefin tuna and a comparison of chromosome structure among teleost species. Adv Biosci Biotechnol 7:85–122
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Friters A, Pot J, Paleman J, Luiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4415
Watanabe T, Yoshida M, Nakajima M, Taniguchi N (2005) Linkage mapping of AFLP and microsatellite DNA markers with the body color- and sex-determining loci in the guppy. Zool Sci 22:883–889
Xiao TQ, Lu CY, Li C, Cheng L, Cao DC, Sun XW (2014) An AFLP-based approach for the identification of sex-linked markers in Amur sturgeon Acipenser schrenckii Brandt, 1869. J Appl Ichthyol 30:1282–1285
Acknowledgements
We thank Dennis Murphy for editing this manuscript.
Funding
This work was supported by JSPS KAKENHI grant numbers JP15H00471 and JP16H00480.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
All experiments described in this manuscript were carried out in accordance with the Guide for the Care and Use of Laboratory Animals from Ehime University.
Rights and permissions
About this article
Cite this article
Sawayama, E., Noguchi, D., Nakayama, K. et al. Identification, Characterization, and Mapping of a Novel SNP Associated with Body Color Transparency in Juvenile Red Sea Bream (Pagrus major). Mar Biotechnol 20, 481–489 (2018). https://doi.org/10.1007/s10126-018-9810-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9810-z