Abstract
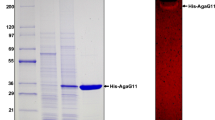
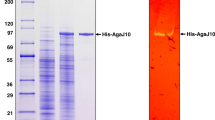
A novel endo-type β-agarase gene, agaA, was cloned from a newly isolated marine bacterium, Agarivorans sp. LQ48. It encodes a protein of 457 amino acids with a calculated molecular mass of 51.2 kDa. The deduced protein contains a typical N-terminal signal peptide of 25 amino acid residues, followed by a catalytic module, which is homologous to that of glycoside hydrolase family 16. A sequence similar to a carbohydrate-binding module is found in the C-terminal region of the enzyme. The overall amino acid sequence shares a highest identity of 73% with the sequence of beta-agarase AgaB from Pseudoalteromonas sp. strain CY24. The mature agarase was highly expressed extracellularly in Escherichia coli. At pH 7.0 and 40°C, the purified recombinant AgaA had a high specific activity of 349.3 μmol min−1 mg−1, a K m of 3.9 mg ml−1, and a V max of 909.1 μmol min−1 mg−1 for agarose. The recombinant enzyme hydrolyzed the β-1,4-glycosidic linkages of agarose, yielding neoagarotetraose and neoagarohexaose as the main products. Enzyme activity analysis revealed that the optimal temperature and pH of the recombinant AgaA were 40°C and 7.0, respectively. Notably, AgaA still retained more than 95% activity after incubation at pH 3.0–11.0 for 1 h, a characteristic much different from other agarases reported. It is the first agarase identified to have so wide a pH range stability. This favorable property could make AgaA to be attractive to the food, cosmetic, and medical industrial applications.






Similar content being viewed by others
References
Araki CH (1937) Acetylation of agar like substance of Gelidium amansii. J Chem Soc Japan 58:1338–1350
Araki T, Hayakawa M, Zhang L, Karita S, Morishita T (1998a) Purification and characterization of agarases from a marine bacterium, Vibrio sp. PO-303. J Mar Biotechnol 6:260–265
Araki T, Lu Z, Morishita T (1998b) Optimization of parameters for isolation of protoplasts from Gracilaria verrucosa (Rhodophyta). J Mar Biotechnol 6:193–197
Dahle H, Garshol F, Madsen M, Birkeland NK (2007) Microbial community structure analysis of produced water from a high-temperature North Sea oil-field. Antonie Van Leeuwenhoek 93:37–49
Dong JH, Tamaru Y, Araki T (2007) A unique β-agarase, AgaA, from a marine bacterium, Vibrio sp. strain PO-303. Appl Microbiol Biotechnol 74:1248–1255
Fu XT, Lin H, Kim SM (2008) Purification and characterization of a novel β-agarase, AgaA34, from Agarivorans albus YKW-34. Appl Microbiol Biotechnol 78:265–273
Hamer GK, Bhattacharjee SS, Yaphe W (1977) Analysis of the enzymic hydrolysis products of agarose by 13C-n.m.r. spectroscopy. Carbohydr Res 54:C7–C10
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Hu Z, Lin BK, Xu Y, Zhong MQ, Liu GM (2009) Production and purification of agarase from a marine agarolytic bacterium Agarivorans sp. HZ105. J Appl Microbiol 106:181–190
Kang NY, Choi YL, Cho YS, Kim BK, Jeon BS, Cha JY, Kim CH, Lee YC (2003) Cloning, expression and characterization of a β-agarase gene from a marine bacterium, Pseudomonas sp. SK38. Biotechnol Lett 25:1165–1170
Kobayashi T, Uchimura K, Miyazaki M, Nogi Y, Horikoshi K (2009) A new high-alkaline alginate lyase from a deep-sea bacterium Agarivorans sp. Extremophiles 13:121–129
Kurahashi M, Yokota A (2004) Agarivorans albus gen. nov., sp. nov., a γ-proteobacterium isolated from marine animals. Int J Syst Evol Microbiol 54:693–697
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lakshmikanth M, Manohar S, Souche Y, Lalitha J (2006) Extracellular β-agarase LSL-1 producing neoagarobiose from a newly isolated agar-liquefying soil bacterium, Acinetobacter sp., AG LSL-1. World J Microbiol Biotechnol 22:1087–1094
Lee DG, Jang MK, Lee OH, Kim NY, Ju SA, Lee SH (2008) Over-production of a glycoside hydrolase family 50 β-agarase from Agarivorans sp. JA-1 in Bacillus subtilis and the whitening effect of its product. Biotechnol Lett 30:911–918
Lee DG, Park GT, Kim NY, Lee EJ, Jang MY, Shin YG, Park GS, Kim TM, Lee JH, Lee JH, Kim SJ, Lee SH (2006) Cloning, expression, and characterization of a glycoside hydrolase family 50 β-agarase from a marine Agarivorans isolate. Biotechnol Lett 28:1925–1932
Ma CP, Lu XZ, Shi C, Li JB, Gu YC, Ma YM, Chu Y, Han F, Gong QH, Yu WG (2007) Molecular cloning and characterization of a novel β-Agarase, AgaB, from marine Pseudoalteromonas sp. CY24. J Biol Chem 282:3747–3754
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Ohta Y, Hatada YJ, Miyazaki M, Nogi YC, Ito S, Horikoshi K (2005) Purification and characterization of a novel α-agarase from a Thalassomonas sp. Current Microbiology 50:212–216
Ohta Y, Nogi YC, Miyazaki M, Miyazaki M, Li Z, Akita M, Hidaka Y, Goda S, Ito S, Horikoshi K (2004a) Enzymatic properties and nucleotide and amino acid sequences of a thermostable β-agarase from the novel marine isolate, JAMB-A94. Biosci Biotechnol Biochem 68:1073–1081
Ohta Y, Hatada Y, Nogi YC, Li Z, Ito S, Horikoshi K (2004b) Cloning, expression, and characterization of a glycoside hydrolase family 86 β-agarase from a deep-sea Microbulbifer-like isolate. Appl Microbiol Biotechnol 66:266–275
Suzuki H, Sawai Y, Suzuki T, Kawai K (2003) Purification and characterization of an extracellular β-Agarase from Bacillus sp. MK03. Journal of Biosci and Bioeng 95:328–334
Syn CK, Swarup S (2000) A scalable protocol for the isolation of large-sized genomic DNA within an hour from several bacteria. Anal Biochem 278:86–90
Wang JX, Mou HJ, Jiang XL, Guan HS (2006) Characterization of a novel β-agarase from marine Alteromonas sp. SY37–12 and its degrading products. Appl Microbiol Biotechnol 71:833–839
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Yoshizawa Y, Ametani A, Tsunehiro J, Nomura K, Itoh M, Fukui F, Kaminogawa S (1995) Macrophage stimulation activity of the polysaccharide fraction from a marine alga (Porphyra yezoensis): structure–function relationships and improved solubility. Biosci Biotechnol Biochem 59:1933–1937
Zhang WW, Sun L (2007) Cloning, characterization, and molecular application of a beta-Agarase gene from Vibrio sp. strain V134. Appl Environ Microbiol 73:2825–2831
Acknowledgment
This work was supported by grant no. 200805050 from the Marine Scientific Research Special Foundation for Public Sector Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Long, M., Yu, Z. & Xu, X. A Novel β-Agarase with High pH Stability from Marine Agarivorans sp. LQ48. Mar Biotechnol 12, 62–69 (2010). https://doi.org/10.1007/s10126-009-9200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9200-7