Abstract
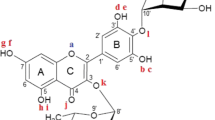
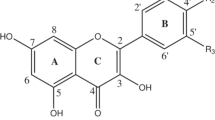
In this work, we present a computational study on the antioxidant potential of myricetin 3-O-α-L-rhamnopyranoside (Compound M3) and myricetin 4′-O-α-L-rhamnopyranoside (Compound M4′). Structural parameters, bond dissociation enthalpies (BDEs), ionization potentials (IPs), proton dissociation enthalpies (PDEs), proton affinities (PAs), and electron transfer enthalpies (ETEs), which are properties connected with different mechanisms related to antioxidant activity, were determined using density functional theory (DFT) with B3LYP, LC-ωPBE, M06-2X, and BMK functionals along with the 6-311G(d,p) and 6-311+G(d,p) basis sets in the gas phase, water, and pentylethanoate. The values obtained were compared with results previously available in the literature for myricetin (the parent molecule and a well-known antioxidant) and myricetin 3,4′-di-O-α-L-rhamnopyranoside (Compound M3,4′). As the BDEs are considerably lower than the IPs, the HAT mechanism is preferred over SET for the compounds M3 and M4′. The present study indicates Compound M3 as having its lowest bond dissociation enthalpy from the several different OH groups with similar value to the lowest for myricetin (74.72 kcal/mol versus 74.8 kcal/mol, respectively, at the B3LYP/6-311G(d,p) level of theory in the gas phase) and, thus, presenting antioxidant potential as good as its parent molecule. On the other hand, Compound M4′ presented 78.97 kcal/mol as the lowest BDE at the B3LYP/6-311G(d,p) level of theory in the gas phase, that is very close to the 78.34 kcal/mol computed using the same approach for Compound M3,4′. Therefore, the present investigation indicated Compound M4′ as being a slightly inferior antioxidant (with antioxidant potential comparable to Compound M3,4′) than Compound M3. In addition, the inclusion of the sugar moiety studied here in the position 4′-ArOH of myricetin seems to have a more marked impact (downward) on the antioxidant activity than the glycosylation in the position 3-ArOH.



Similar content being viewed by others
References
Stavrou IJ, Christou A, Kapnissi-Christodoulou CP (2018) Polyphenols in carobs: a review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem 269:355–174
Minnaar P, Nyobo L, Jolly N, Ntushelo N, Meiring S (2018) Anthocyanins and polyphenols in Cabernet Franc wines produced with Saccharomyces cerevisiae and Torulaspora delbrueckii yeast strains: spectrophotometric analysis and effect on selected sensory attributes. Food Chem 268:287–291
Bilgin M, Elhussein EAA, Ozyurek M, Guclu K, Sahin S (2018) Optimizing the extraction of polyphenols from Sideritis montana L. using response surface methodology. J Pharm Biomed Anal 158:137–143
Liu SW, You L, Zhao YX, Chang XD (2018) Hawthorn polyphenol extract inhibits UVB-induced skin photoaging by regulating MMP expression and type I procollagen production in mice. J Agric Food Chem 66:8537–8546
Riaz A, Lei SC, Akhtar HMS, Wan P, Chen D, Jabbar S, Abid M, Hashim MM, Zeng XX (2018) Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int J Biol Macromol 114:547–555
Silva CP, Sampaio GR, Freitas RAMS, Torres EAFS (2018) Polyphenols from guarana after in vitro digestion: evaluation of bioaccessibility and inhibition of activity of carbohydrate-hydrolyzing enzymes. Food Chem 267:405–409
Shahidi F, Ambigaipalan P (2015) Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects—a review. J Funct Foods 18:820–897
Guajardo-Flores D, Serna-Saldivar SO, Gutiérrez-Uribe JA (2013) Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.) Food Chem 141:1497–1503
Plaza M, Kariuki J, Turner C (2014) Quantification of individual phenolic compounds: contribution to antioxidant capacity in apple: a novel analytical tool based on liquid chromatography with diode array, electrochemical, and charged aerosol detection. J Agric Food Chem 62:409–418
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem A 108:92–96
Zhang XC, Chen F, Wang MF (2014) Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J Agric Food Chem 62:1643–1648
Aswathy VV, Alper-Hayta S, Yalcin G, Mary YS, Panicker CY, Jojo PJ, Kaynak-Onurdag F, Armaković S, Armaković SJ, Yildiz I, Alsenoy CV (2017) Modification of benzoxazole derivative by bromine-spectroscopic, antibacterial and reactivity study using experimental and theoretical procedures. J Mol Struct 1141:495–511
Belščak-Cvitanović A, Durgo K, Bušić A, Franekić J, Komes D (2014) Phytochemical attributes of four conventionally extracted medicinal plants and cytotoxic evaluation of their extracts on human laryngeal carcinoma (HEp2) cells. J Med Food 17:206–217
Malig TC, Ashkin MR, Burman AL, Barday M, Heyne BJ, Back TG (2017) Comparison of free-radical inhibiting antioxidant properties of carvedilol and its phenolic metabolites. Med Chem Comm 8:606–615
Ren F, Reilly K, Kerry JP, Gaffney M, Hossain M, Rai DK (2017) Higher antioxidant activity, total flavonols, and specific quercetin glucosides in two different onion (Allium cepa L.) varieties grown under organic production: results from a 6-year field study. J Agric Food Chem 65:5122–5132
Li X, Liu J, Lin J, Wang T, Huang J, Lin Y, Chen D (2016) Protective effects of dihydromyricetin against OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 21:604
Tedesco I, Carbone V, Spagnuolo C, Minasi P, Russo GL (2015) Identification and quantification of flavonoids from two southern Italian cultivars of Allium cepa L., Tropea (red onion) and Montoro (copper onion), and their capacity to protect human erythrocytes from oxidative stress. J Agric Food Chem 63:5229–5238
Fliniaux O, Corbin C, Ramsay A, Renouard S, Beejmohun V, Doussot J, Falguieres A, Ferroud C, Lamblin F, Laine E, Roscher A, Grand E, Mesnard F, Hano C (2014) Microwave-assisted extraction of herbacetin diglucoside from flax (Linum usitatissimum L.) seed cakes and its quantification using an RP-HPLC-UV system. Molecules 19:3025–3037
Guajardo-Flores D, Serna-Saldivar SO, Gutiérrez-Uribe JA (2013) Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem 141:1497–1503
Wang C, Yin YH, Wei YJ, Shi ZQ, Liu JQ, Liu LF, Xin GZ (2017) Rapid identification of herbal compounds derived metabolites using zebrafish larvae as the biotransformation system. J Chromatogr A 1515:100–108
Chirug L, Okun Z, Ramon O, Shpigelman A (2018) Iron ions as mediators in pectin-flavonols interactions. Food Hydrocoll 84:441–449
Fan GH, Zhu S, Xu H (2018) Density-functional theory study of the interaction mechanism and optical properties of flavonols on the boron nitride nanotubes. Int J Quantum Chem 118:e25514
Falantin C, Moncomble A, Le Person A, Cornard JP (2017) Chalcogen substitution: effect of oxygen-by-sulfur exchange on structural and spectroscopic properties of flavonols. Spectrochim Acta A 187:49–60
de Souza GLC, de Oliveira LMF, Vicari RG, Brown A (2016) A DFT investigation on the structural and antioxidant properties of new isolated interglycosidic O-(1→3) linkage flavonols. J Mol Model 22:100–109
Mendes RA, e Silva BLS, Takeara R, Freitas RG, Brown A, de Souza GLC (2018) Probing the antioxidant potential of phloretin and phlorizin through a computational investigation. J Mol Model 24:101
Maciel EN, Almeida SKC, da Silva SC, de Souza GLC (2018) Examining the reaction between antioxidant compounds and 2,2-diphenyl-1-picrylhydrazyl (DPPH) through a computational investigation. J Mol Model 24:218
Sandoval-Yanez C, Mascayano C, Martinez-Araya JI (2018) A theoretical assessment of antioxidant capacity of flavonoids by means of local hyper-softness. Arab J Chem 11:554–563
Garcia G, Atilhan M, Aparicio S (2016) Flavonol-carbon nanostructure hybrid systems: a DFT study on the interaction mechanism and UV/Vis features. Phys Chem Chem Phys 18:4760–4771
Yao Y, Lim G, Xie Y, Ma P, Li G, Meng Q, Wu T (2014) Preformulation studies of myricetin: a natural antioxidant flavonoid. Pharmazie 69:19–26
Gordon MH, Roedig-Penman A (1998) Antioxidant activity of quercetin and myricetin in liposomes. Chem Phys Lipids 97:79–85
Chobot V, Hadacek F (2011) Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep 16:242–247
Nasri I, Chawech R, Girardi C, Mas E, Ferrand A, Vergnolle N, Fabre N, Mezghani-Jarraya R, Racaud-Sultan C (2017) Anti-inflammatory and anticancer effects of flavonol glycosides from Diplotaxis harra through GSK3 beta regulation in intestinal cells. Pharm Biol 55:124–131
Bell L, Oruna-Concha MJ, Wagstaff C (2015) Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: highlighting the potential for improving nutritional value of rocket crops. Food Chem 172:852–861
Grzesik M, Bartosz G, Dziedzic A, Narog D, Namiesnik J, Sadowska-Bartosz I (2018) Antioxidant properties of ferrous flavanol mixtures. Food Chem 268:567–576
Giacomelli C, Miranda F da S, Goncalves NS, Spinelli A (2004) Antioxidant activity of phenolic and related compounds: a density functional theory study on the O-H bond dissociation enthalpy. Redox Rep 9:263–269
Galato D, Giacomelli C, Ckless K, Susin MF, Vale RM4R, Spinelli A (2001) Antioxidant capacity of phenolic and related compounds: correlation among electrochemical, visible spectroscopy methods and structure–antioxidant activity. Redox Rep 6:243–250
Chen Y, Xiao H, Zheng J, Liang G (2015) Structure–thermodynamics–antioxidant activity relationships of selected natural phenolic acids and derivatives: an experimental and theoretical evaluation. PLoS One 10:e0121276
Zheng Y-Z, Chen D-F, Deng G, Guo R, Fu Z-M (2018) The surrounding environments on the structure and antioxidative activity of luteolin. J Mol Model 24:149
Musialik M, Kuzmicz R, Pawlowski TS, Litwinienko G (2009) Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J Org Chem 74:2699–2709
Musialik M, Litwinienko G (2005) Scavenging of dpph radicals by vitamin E is accelerated by its partial ionization: the role of sequential proton loss electron transfer. Org Lett 7:4951– 4954
Zhang Z, ElSohly HN, Li X C, Khan SI, Broedel SE Jr, Raulli RE, Cihlar RL, Burandt C, Walker LA (2003) Phenolic compounds from Nymphaea odorata. J Nat Prod 66:548–550
Isidoro MM, da Silva MFGF, Fernandes JB, Vieira PC, Arruda AC, Silva SC (2012) Phytochemical and chemosystematic studies of Euxylophora paraensis (Rutaceae). Quim Nova 35:2119–2124
Sidana J, Neeradi D, Choudhary A, Singh S, Foley WJ, Singh IP (2013) Antileishmanial polyphenols from Corymbia maculata. J Chem Sci 125:765–775
Kishore N, Twilley D, Blom van Staden A, Verma P, Singh B, Cardinali G, Kovacs D, Picardo M, Kumar V, Lall N (2018) Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J Nat Prod 81:49–56
Sadasivam K, Kumaresan R (2011) Antioxidant behavior of mearnsetin and myricetin flavonoid compounds—a DFT study. Spectrochim Acta A 79:282–293
Li M-J, Liu L, Fu Y, Guo Q-X (2007) Accurate bond dissociation enthalpies of popular antioxidants predicted by the ONIOM-G3B3 method. J Mol Struct: THEOCHEM 815:1–9
Mendes RA, Almeida SKC, Soares IN, Barboza CA, Freitas RG, Brown A, de Souza GLC (2018) A computational investigation on the antioxidant potential of myricetin 3,4′-di-O-α-L-rhamnopyranoside. J Mol Model 24:133
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Vydrov OA, Scuseria GE (2006) Assessment of a long range corrected hybrid functional. J Chem Phys 125:234109
Rassolov V, Pople JA, Ratner M, Redfern PC, Curtiss LA (2001) 6-31G* basis set for third-row atoms. J Comp Chem 22:976– 984
Binkley JS, Pople JA, Hehre WJ (1980) Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J Am Chem Soc 102:939–946
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261
Zhao Y, Truhlar DG (2006) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Boese AD, Martin JML (2004) Development of novel density functionals for thermochemical kinetics. J Chem Phys 121:3405
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum models of solvation. I. General formalism. J Chem Phys 132:114110
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041
Bartmess JE (1994) Thermodynamics of the electron and the proton. J Phys Chem 98:6420–6424
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin K N, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT, Gaussian 09, Revision D.01
Vojta D, Dominkocić K, Miljanić S, Spanget-Larsen J (2017) Intramolecular hydrogen bonding in myricetin and myricitrin. Quantum chemical calculations and vibrational spectroscopy. J Mol Struct 1131:242–249
Gazquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A 111:1966–1970
Martinez A (2009) Donator acceptor map of psittacofulvins and anthocyanins: are they good antioxidant substances. J Phys Chem B 113:4915–4921
Johns JR, Platts JA (2014) Theoretical insight into the antioxidant properties of melatonin and derivatives. Org Biomol Chem 12:7820
Justino GC, Vieira AJSC (2010) Antioxidant mechanisms of quercetin and myricetin in the gas phase and in solution—a comparison and validation of semi-empirical methods. J Mol Model 16:863–876
Acknowledgements
The Brazilian agency CNPq funded this work (Process number: 306266/2016-4). AB thanks the Natural Sciences and Engineering Research Council of Canada for funding (NSERC - Discovery Grant). This research was supported in part by PL-Grid Infrastructure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Cartesian coordinates for optimized geometries, harmonic frequencies for the OH stretches, and the spin densities for the various radicals can be found in the Supplementary Material.
This paper belongs to the Topical Collection VII Symposium on Electronic Structure and Molecular Dynamics – VII SeedMol
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mendes, R.A., Almeida, S.K.C., Soares, I.N. et al. Evaluation of the antioxidant potential of myricetin 3-O-α-L-rhamnopyranoside and myricetin 4′-O-α-L-rhamnopyranoside through a computational study. J Mol Model 25, 89 (2019). https://doi.org/10.1007/s00894-019-3959-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-3959-x