Abstract
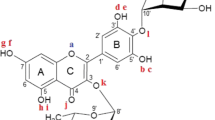
The structures and energetics of two dihydrochalcones (phloretin and its glycoside phlorizin) were examined with density functional theory, using the B3LYP, M06-2X, and LC-ω PBE functionals with both the 6-311G(d,p) and 6-311 + G(d,p) basis sets. Properties connected to antioxidant activity, i.e., bond dissociation enthalpies (BDEs) for OH groups and ionization potentials (IPs), were computed in a variety of environments including the gas-phase, n-hexane, ethanol, methanol, and water. The smallest BDEs among the four OH groups for phloretin (three for phlorizin) were determined (using B3LYP/6-311 + G(d,p) in water) to be 79.36 kcal/mol for phloretin and 79.98 kcal/mol for phlorizin while the IPs (at the same level of theory) were obtained as 139.48 and 138.98 kcal/mol, respectively. By comparing with known antioxidants, these values for the BDEs indicate both phloretin and phlorizin show promise for antioxidant activity. In addition, the presence of the sugar moiety has a moderate (0-6 kcal/mol depending on functional) effect on the BDEs for all OH groups. Interestingly, the BDEs suggest that (depending on the functional chosen) the sugar moiety can lead to an increase, decrease, or no change in the antioxidant activity. Therefore, further experimental tests are encouraged to understand the substituent effect on the BDEs for phloretin and to help determine the most appropriate functional to probe BDEs for dihydrochalcones.

Similar content being viewed by others
References
Fernandez-Pastor I, Fernandez-Hernandez A, Rivas F, Martinez A, Garcia-Granados A, Parra A (2016) Synthesis and antioxidant activity of hydroxytyrosol Alkyl-Carbonate derivatives. J Nat Prod 79:1737–1745
Wu G, Johnson SK, Bornman JF, Bennett SJ, Fang Z (2017) Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem 214:199–207
Murthy PK, Mary YS, Suneetha V, Panicker CY, Armaković S, Armaković SJ, Giri L, Suchetan PA, Alsenoy CV (2017) Towards the new heterocycle based molecule: synthesis, characterization and reactivity study. J Mol Struct 1137:589–605
Varnali T (2016) A comparison of scytonemin and its carbon analogue in terms of antioxidant properties through free radical mechanisms and conformational analysis: a DFT investigation. J Mol Model 22:213
Grajeda-Iglesias C, Figueroa-Espinoza MC, Barouh N, Baréa B, Fernandes A, de Freitas V, Salas E (2016) Isolation and characterization of anthocyanins from hibiscus sabdariffa flowers. J Nat Prod 79:1709–1718
Okumura K, Hosoya T, Kawarazaki K, Izawa N, Kumazawa S (2016) Antioxidant activity of phenolic compounds from fava bean sprouts. J Food Sci 81:1394–1398
Benzon KB, Sheena MY, Panicker CY, Armaković S, Armaković SJ, Pradhan K, Nanda AK, Alsenoy CV (2017) Studies on the synthesis, spectroscopic analysis, molecular docking and DFT calculations on 1-hydroxy-2-(4-hydroxyphenyl)-4,5-dimethyl-imidazol 3-oxide. J Mol Struct 1130:644–658
Ranjith PK, Mary YS, Panicker CY, Anto PL, Armaković S, Armaković SJ, Musiol R, Jampilek J, Alsenoy CJ (2017) New quinolone derivative: spectroscopic characterization and reactivity study by DFT and MD approaches. J Mol Struct 1135:1–14
Apak R, Ozyurek M, Guclu K, Capanoglu E (2016) Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Afric Food Chem 64:997–1027
Malig TC, Ashkin MR, Burman AL, Barday M, Heyne BJ, Back TG (2017) Comparison of free-radical inhibiting antioxidant properties of carvedilol and its phenolic metabolites. Med Chem Comm 8:606–615
Rahman A-U (2016) Studies in natural products chemistry, vol 51. Elsevier, Oxford, pp 253–373
Aswathy VV, Alper-Hayta S, Yalcin G, Mary YS, Panicker CY, Jojo PJ, Kaynak-Onurdag F, Armaković S, Armaković SJ, Yildiz I, Alsenoy CV (2017) Modification of benzoxazole derivative by bromine-spectroscopic, antibacterial and reactivity study using experimental and theoretical procedures. J Mol Struct 1141:495–511
Plaza M, Kariuki J, Turner C (2014) Quantification of individual phenolic compounds’ contribution to antioxidant capacity in apple: a novel analytical tool based on liquid chromatography with diode array, electrochemical, and charged aerosol detection. J Agric Food Chem 62:409–418
Zhang XC, Chen F, Wang MF (2014) Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J Agric Food Chem 62:1643–1648
Belščak-Cvitanović A, Durgo K, Bušić A, Franekić J, Komes D (2014) Phytochemical attributes of four conventionally extracted medicinal plants and cytotoxic evaluation of their extracts on human laryngeal carcinoma (HEp2) cells. J Med Food 17:206–217
Nakamura Y, Watanabe S, Miyake N, Kohno H, Osawa T (2003) Dihydrochalcones: evaluation as novel radical scavenging antioxidants. J Agric Food Chem 51:3309–3312
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of Apigenin, Luteolin, and Taxifolin antioxidants. a first principle theoretical study. J Phys Chem A 108:92–96
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-Atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922
Leopoldini M, Marino T, Russo N, Toscano M (2004) Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas-phase and in solvent. Theor Chem Acc 111:210–216
Apak R, Ozyurek M, Guclu K, Capanoglu E (2016) Antioxidant activity/capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid Peroxidation assays. J Agric Food Chem 64:1028–1045
Nenadis N, Sigalas MP (2008) A DFT study on the radical scavenging activity of maritimetin and related aurones. J Phys Chem A 112:12196–12202
Guajardo-Flores D, Serna-Saldivar SO, Gutiérrez-Uribe J A (2013) Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus Vulgaris L.) Food Chem 141:1497–1503
Stepanić V, Troselj KG, Lucić B, Marković Z, Amić D (2013) Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem 141:1562–1570
Wright JS, Carpenter DJ, McKay DJ, Ingold KU (1997) Theoretical calculation of substituent effects on the O-H bond strength of phenolic antioxidants related to vitamin E. J Am Chem Soc 119:4245–4252
Brinck T, Lee H -N, Jonsson M (1999) Quantum chemical studies on the thermochemistry of alkyl and peroxyl radicals. J Phys Chem A 103:7094–7104
Gomes JRB, da Silva MAVR (2003) Gas-phase thermodynamic properties of dichlorophenols determined from density functional theory calculations. J Phys Chem A 107:869–874
Vagánek A, Rimarčik J, Lukeš V, Klein E (2012) On the energetics of homolytic and heterolytic O-H bond cleavage in flavonols. Comput Theor Chem 991:192–200
Vagánek A, Rimarčik J, Dropkova K, Lengyel J, Klein E (2014) Reaction enthalpies of O–H bonds splitting-off in flavonoids: the role of non-polar and polar solvent. Comput Theor Chem 1050:31–38
Trouillas P, Marsal P, Siri D, Lazzaroni R, Duroux J -L (2006) A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: the specificity of the 3-OH site. Food Chem 97:679–688
Amić D, Stepanić W, Lučić R, Marković Z, Dmitrić Marković JM (2013) PM6 Study of free radical scavenging mechanisms of flavonoids: why does OH bond dissociation enthalpy effectively represent free radical scavenging activity. J Mol Model 19:2593–2603
Justino GC, Vieira AJSC (2010) Antioxidant mechanisms of Quercetin and Myrcetin in the gas pase and in solution - a comparison and validation of semi-empirical methods. J Mol Model 16:863–876
Antonczak A (2008) Electronic description of four flavonoids revisited by DFT method. J Mol Struct: THEOCHEM 856:38–45
Li M -J, Liu L, Fu Y, Guo Q -X (2007) Accurate bond dissociation enthalpies of popular antioxidants predicted by the ONIOM-g3b3 method. J Mol Struct: THEOCHEM 815:1–9
Lengyel J, Rimarčik J, Vagánek A, Klein E (2013) On the radical scavenging activity of isoflavones: thermodynamics of O-H bond cleavage. Phys Chem Chem Phys 15:10895–10903
Cai W, Chen Y, Xie L, Zhang H, Hou C (2014) Characterization and density functional theory study of the antioxidant activity of quercetins and its sugar-containing analogues. Eur Food Res Technol 238:121–128
Lespade L, Bersion S (2012) Theoretical investigation of the effect of sugar substitution on the antioxidant properties of flavonoids. Free Radic Res 46:346–358
Deepha V, Praveena R, Sivakumar R, Sadasivam K (2014) Experimental and theoretical investigations on the antioxidant activity of isoorientin from Crotalaria globosa. Spectrochim Acta A 121:737–745
Mohajeri A, Asemani SS (2009) Theoretical investigation on antioxidant activity of vitamins and phenolic acids for designing a novel antioxidant. J Mol Struct 930:15–20
de Souza GLC, de Oliveira LMF, Vicari RG, Brown A (2016) A DFT investigation on the structural and antioxidant properties of new isolated interglycosidic O-(1 → 3) linkage flavonols. J Mol Model 22:100–109
Auner BG, Valenta C, Hadgraft J (2003) Influence of phloretin and 6-Ketocholestanol on the skin permeation of Sodium-Fluorescein. J Control Release 89:321–328
Blazso G, Gabor M (1995) Effects of prostaglandin antagonist phloretin derivatives on mouse ear edema induced with different skin irritants. Prostaglandis 50:161–168
Oresajo C (2008) Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid and phloretin against ultraviolet-induced photodamage in human skin. J Cosmet Dermatol 7:290–297
Rezk BM, Haenen GRMM, van der Vijgh WJF, Bast A (2002) The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem Biophys Res Commun 295:9–13
Zhang S, Morris ME (2003) Effects of the flavonoids Biochanin A, Morin, Phloretin, and Silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther 304:1258–1267
Park SY, Kim EJ, Shin HK, Kwon DY, Kim MS, Surh YJ, Park JH (2007) Induction of apoptosis in HT-29 colon cancer cells by phloretin. J Med Food 10:581–586
Luo H, Wang YJ, Liu JQ (2008) Study on the effect of phloretin on inhibiting malignant pheotype of BEL-7402 cells. Zhong Yao Cai 31:1019–1021
Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY (2009) In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer 124:2210– 2219
Nithyia T, Udayakumar R (2016) In vitro antioxidant properties of Phloretin—an important phytocompound. J Biosci Med 4:85– 94
Kozlowski D, Trouillas P, Calliste C, Marsal P, Lazzaroni R, Duroux J-L (2007) Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J Phys Chem A 111:1138–1145
Yan M, Gong J, Shen P, Yang C (2014) The theory investigation for the antioxidant activity of phloretin: a comparation with naringenin. AMM 513:359–362
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200– 1211
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Rassolov V, Pople JA, Ratner M, Redfern PC, Curtiss LA (2001) 6-31G* basis set for third-row atoms. J Comput Chem 22:976– 984
Binkley JS, Pople JA, Hehre WJ (1980) Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J Am Chem Soc 102:939–946
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Hehre WJ, Ditchfield R, Pople JA (1972) Self-Consistent Molecular orbital methods. XII. Further extensions of Gaussian-Type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261
La Rocca MV, Rutkowski M, Ringeissen S, Gomar J, Frantz M-C, Ngom S, Adamo C (2016) Benchmarking the DFT methodology for assessing antioxidant-related properties: quercetin and edaravone as case studies. J Mol Model 22:250–260
Zhao Y, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2006) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215–241
Vydrov OA, Scuseria GE (2006) Assessment of a long range corrected hybrid functional. J Chem Phys 125:234109
Aufmkolk M, Koehrle J, Hesch R-D, Ingbar SH, Cody V (1986) Crystal structure of phlorizin and the iodothyronine deiodinase inhibitory activity of phloretin analogues. Biochem Pharmacol 35:2221–2227
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum models of solvation. I. General formalism. J Chem Phys 132:114110
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041
Mennucci B, Cancès E, Tomasi J (1997) Evaluation of solvent effects in isotropic and anisotropic dielectrics, and in ionic solutions with a unified integral equation method: theoretical bases, computational implementation and numerical applications. J Phys Chem B 101:10506–10517
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT, Gaussian 09, Revision D.01
Brinck T, Haeberlein M, Jonsson M (1997) A computational analysis of substituent effects on the O-H bond dissociation energy in phenols: polar versus radical effects. J Am Chem Soc 119:4239–4244
Kalita D, Kar R, Handique JG (2012) A theoretical study on the antioxidant property of gallic acid and its derivatives. J Theory Comput Chem 11:391–402
Paya M, Goodwin PA, De las Heras B, Hoult JRS (1994) Superoxide scavenging activity in leukocytes and absence of cellular toxicity of a series of coumarins. Biochem Pharmacol 48:445–451
Souza LP, Calegari F, Zarbin AJG, Marcolini-Júnior LH, Bergamini MF (2011) Voltammetric determination of the antioxidant capacity in wine samples using a carbon nanotube modified electrode. J Agric Food Chem 59:7620–7625
Hernández-Herrero JA, Frutos MJ (2014) Colour and antioxidant capacity stability in grape, strawberry and plum peel model juices at different pHs and temperatures. Food Chem 154:199– 204
Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Acknowledgments
GLCS thanks Dr. Mariana Pantoja, MD from the Universidade do Estado do Amazonas for useful discussions. This work was partially funded by the Brazilian agency CNPq (Process number: 306266/2016-4). AB thanks the Natural Sciences and Engineering Research Council of Canada for funding (NSERC - Discovery Grant).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mendes, R.A., e Silva, B.L.S., Takeara, R. et al. Probing the antioxidant potential of phloretin and phlorizin through a computational investigation. J Mol Model 24, 101 (2018). https://doi.org/10.1007/s00894-018-3632-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3632-9