Abstract
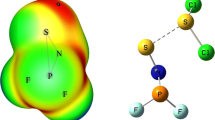
Ab initio calculations have been carried out to investigate the σ-hole interaction in XHY···CH3 and XHY···CH2CH3 complexes, where X = F, Cl, Br and Y = S, Se. This interaction, termed “single-electron chalcogen bond interaction” was analyzed in terms of geometric, interaction energies and electronic features of the complexes. This interaction is a weak one, with an interaction energy that varies from a minimum of -1.7 kcal mol-1 for BrHS···CH3 to -6.0 kcal mol-1 for FHSe···CH2CH3 at the CCSD(T)/aug-cc-pVTZ level of theory. Energy decomposition analysis indicated that the dominant attraction energy originates in the electrostatic term which is larger for the Se complexes than for the S counterparts. However, the attractive polarization and dispersion components also make an important contribution to the interaction energy for the single-electron chalcogen bond interactions.





Similar content being viewed by others
References
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Esrafili MD, Behzadi H, Hadipour NL (2008) 14N and 17O electric field gradient tensors in benzamide clusters: theoretical evidence for cooperative and electronic delocalization effects in N–H···O hydrogen bonding. Chem Phys 348:175–180
Esrafili MD, Behzadi H, Hadipour NL (2008) Density functional theory study of N–H⋯ O, O–H⋯ O and C–H⋯ O hydrogen-bonding effects on the 14 N and 2 H nuclear quadrupole coupling tensors of N-acetyl-valine. Biophys Chem 133:11–18
Berka K, Laskowski R, Riley KE, Hobza P, Vondrášek J (2009) Representative amino acid side chain interactions in proteins. A comparison of highly accurate correlated ab initio quantum chemical and empirical potential procedures. J Chem Theory Comput 5:982–992
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264
Kim KS, Tarakeshwar P, Lee JY (2000) Molecular clusters of π-systems: theoretical studies of structures, spectra, and origin of interaction energies. Chem Rev 100:4145–4186
Wang BQ, Li ZR, Wu D, Hao XY, Li RJ, Sun CC (2003) Single-electron hydrogen bonds in the methyl radical complexes H3C⋯HF and H3C⋯HCCH: an ab initio study. Chem Phys Lett 375:91–95
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc Chem Res 38:386–395
Politzer P, Murray JS, Concha MC (2007) Halogen bonding and the design of new materials: organic bromides, chlorides and perhaps even fluorides as donors. J Mol Model 13:643–650
Esrafili MD, Solimannejad M (2013) Revealing substitution effects on the strength and nature of halogen-hydride interactions: a theoretical study. J Mol Model 19:3767–3777
Esrafili MD (2013) A theoretical investigation of the characteristics of hydrogen/halogen bonding interactions in dibromo-nitroaniline. J Mol Model 19:1417–1427
Esrafili MD, Esmailpour P, Mohammadian F, Solimannejad M (2013) Theoretical study of the interplay between halogen bond and lithium–π interactions: cooperative and diminutive effects. Chem Phys Lett 588:47–50
Politzer P, Lane P, Concha MC, Ma YG, Murray JS (2007) An overview of halogen bonding. J Mol Model 13:305–311
Murray JS, Lane P, Clark T, Politzer P (2007) σ‐Hole bonding: molecules containing group VI atoms. J Mol Model 13:1033–1038
Murray JS, Lane P, Politzer P (2008) Simultaneous σ-hole and hydrogen bonding by sulfur- and selenium-containing heterocycles. Int J Quantum Chem 108:2770–2781
Riley KE, Murray JS, Politzer P, Concha MC, Hobza P (2009) Br···O complexes as probes of factors affecting halogen bonding: interactions of bromobenzenes and bromopyrimidines with acetone. J Chem Theory Comput 5:155–163
Politzer P, Murray JS, Clark T (2010) Halogen bonding: an electrostatically-driven highly directional noncovalent interaction. Phys Chem Chem Phys 12:7748–7757
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) σ-Holes, π-holes and electrostatically-driven interactions. J Mol Model 18:541–548
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Perspectives on halogen bonding and other σ-hole interactions: lex parsimoniae (Occam’s Razor). Comput Theor Chem 998:2–8
Politzer P, Murray JS (2013) Halogen bonding: an interim discussion. ChemPhysChem 14:278–294
Riley KE, Murray JS, Fanfrlík J, Řezáč J, Solá RJ, Concha MC, Ramos FM, Politzer P (2013) Halogen bond tunability II: the varying roles of electrostatic and dispersion contributions to attraction in halogen bonds. J Mol Model 19:4651–4659
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Politzer P, Murray JS, Janjić GV, Zarić SD (2013) σ-Hole interactions of covalently-bonded nitrogen, phosphorus and arsenic: a survey of crystal structures. Crystal 4:12–31
Bleiholder C, Werz DB, Köppel H, Gleiter R (2006) Theoretical investigations on chalcogen-chalcogen interactions: what makes these nonbonded interactions bonding? J Am Chem Soc 128:2666–2674
Wang W, Ji B, Zhang Y (2009) Chalcogen bond: a sister noncovalent bond to halogen bond. J Phys Chem A 113:8132–8135
Politzer P, Murray JS, Clark T (2014) σ-Hole bonding: a physical interpretation. Top Curr Chem. doi:10.1007/128_2014_568
Politzer P, Murray JS (2009) In: Leszczynski J, Shukla M (eds) Practical aspects of computational chemistry. Springer, Heidelberg, p 149
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Iwaoka M, Takemoto S, Tomoda S (2002) Statistical and theoretical investigations on the directionality of nonbonded S···O interactions. Implications for molecular design and protein engineering. J Am Chem Soc 124:10613–10620
Saczewski J, Frontera A, Gdaniec M, Brzozowski Z, Saczewski F, Tabin P, Quiñonero D, Deyà PM (2006) Synthesis, X-ray structure analysis and computational studies of novel bis(thiocarbamoyl) disulfides with non-covalent S⋯N and S⋯S interactions. Chem Phys Lett 422:234–239
Zhang Y, Wang W (2009) The bifurcate chalcogen bond: some theoretical observations. J Mol Struct THEOCHEM 916:135–138
Esrafili MD, Vakili M (2014) Cooperativity effects between σ-hole interactions: a theoretical evidence for mutual influence between chalcogen bond and halogen bond interactions in F2S∙∙∙NCX∙∙∙NCY complexes (X = F, Cl, Br, I; Y = H, F, OH). Mol Phys 112:2746–2752
Esrafili MD, Vakili M (2014) Halogen bonds enhanced by σ-hole and π-hole interactions: a comparative study on cooperativity and competition effects between X∙∙∙N and S∙∙∙N interactions in H3N∙∙∙XCN∙∙∙SF2 and H3N∙∙∙XCN∙∙∙SO2 complexes (X = F, Cl, Br and I). J Mol Model 20:2291
Li QZ, Li R, Guo P, Li H, Li WZ, Cheng JB (2012) Competition of chalcogen bond, halogen bond, and hydrogen bond in SCS–HOX and SeCSe–HOX (X = Cl and Br) complexes. Comput Theor Chem 980:56–61
Bauzá A, Quiñonero D, Deyà PM, Frontera A (2013) Halogen bonding versus chalcogen and pnicogen bonding: a combined Cambridge structural database and theoretical study. CrystEngComm 15:3137–31440
Brezgunova ME, Lieffrig J, Aubert E, Dahaoui S, Fertey P, Lebègue S, Ángyán JG, Fourmigué M, Espinosa E (2013) Chalcogen bonding: experimental and theoretical determinations from electron density analysis. Geometrical preferences driven by electrophilic — nucleophilic interactions. Cryst Growth Des 13:3283–3289
Scheiner S (2013) Detailed comparison of the pnicogen bond with chalcogen, halogen, and hydrogen bonds. Int J Quantum Chem 113:1609–1620
Li Q, Hui Q, Li R, Liu X, Li W, Cheng J (2012) Prediction and characterization of a chalcogen–hydride interaction with metal hybrids as an electron donor in F2CS–HM and F2CSe–HM (M = Li, Na, BeH, MgH, MgCH3) complexes. Phys Chem Chem Phys 14:3025–3030
Wang Y, Zou J, Lu Y, Yu Q, Xu H (2007) Single-electron halogen bond: ab initio study. Int J Quantum Chem 107:501–506
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Su P, Li H (2009) Energy decomposition analysis of covalent bonds and intermolecular interactions. J Chem Phys 131:014102
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691
Biegler-Konig F, Schonbohm J, Bayles D (2001) AIM 2000. J Comput Chem 22:545–559
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) Properties of atoms in molecules: atomic volumes. J Am Chem Soc 109:7968–7979
Hix S, Kadiis MB, Mason RP, Augusto O (2000) In vivo metabolism of tert-butyl hydroperoxide to methyl radicals. EPR spin-trapping and DNA methylation studies. Chem Res Toxicol 13:1056–1064
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Hobza P, Zahradnik R, Muller-Dethlefs K (2006) The world of non-covalent interactions. Collect Czechoslov Chem Commun 71:443–531
Clark T, Politzer P, Murray JS (2015) Correct electrostatic treatment of noncovalent interactions: the importance of polarization. Wires Comput Mol Sci. doi:10.1002/wcms.1210
Riley KE, Murray JS, Fanfrlík J, Řezáč J, Solá RJ, Concha MC, Ramos FM, Politzer P (2013) Halogen bond tunability II: the varying roles of electrostatic and dispersion contributions to attraction in halogen bonds. J Mol Model 19:4651–4659
Koch U, Popelier PLA (1995) Characterization of C-H···O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754
Esrafili MD, Mohammadian F (2015) Ab initio calculations of cooperativity effects on chalcogen bonding: linear clusters of (OCS)2–8 and (OCSe) 2–8. Struct Chem 26:199–206
Esrafili MD, Mahdavinia G, Javaheri M, Sobhi HR (2014) A theoretical study of substitution effects on halogen–π interactions. Mol Phys 112:1160–1166
Clark T, Murray JS, Politzer P (2013) Role of polarization in halogen bonds. Aust J Chem 67:451–456
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Indicates the optimized structure of XHY···CH3 and XHY···CH2CH3 complexes (X=F, Cl, Br; Y=S, Se). (DOC 2220 kb)
Rights and permissions
About this article
Cite this article
Esrafili, M.D., Mohammadian-Sabet, F. Does single-electron chalcogen bond exist? Some theoretical insights. J Mol Model 21, 65 (2015). https://doi.org/10.1007/s00894-015-2613-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2613-5