Abstract
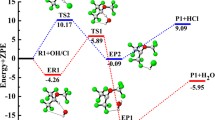
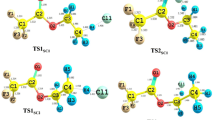
A Theoretical study on the mechanism of the reactions of CF2ClC(O)OCH3 with the OH radical and Cl atom is presented. Geometry optimization and frequency calculations have been performed at the MPWB1K/6-31+G(d,p) level of theory and energetic information is further refined by calculating the energy of the species using G2(MP2) theory. Transition states are searched on the potential energy surface involved during the reaction channels and each of the transition states are characterized by presence of only one imaginary frequency. The existence of transition states on the corresponding potential energy surface is ascertained by performing intrinsic reaction coordinate (IRC) calculation. Theoretically calculated rate constants at 298 K and atmospheric pressure using the canonical transition state theory (CTST) are found to be in good agreement with the experimentally measured ones. Using group-balanced isodesmic reactions as working chemical reactions, the standard enthalpies of formation for CF2ClC(O)OCH3, CF2ClC(O)OCH2 and CF3C(O)OCH3 are also reported for the first time.


Similar content being viewed by others
References
Molina MJ, Rowland FS (1974) Nature 249:810–814
Farman JD, Gardiner BG, Shanklin JD (1985) Nature 315:207–210
Tsai WT (2005) J Hazard Mater 119:69–78
Sekiya A, Misaki S (2000) J Fluorine Chem 101:215–221
Sherwood GJ (2000) US Patent No-6,148,634
Tucker MK (2012) Proceedings of The National Conference On Undergraduate Research (NCUR) Weber State University, Ogden P. No. 29–31
Ravishankara RA, Turnipseed AA, Jensen NR, Barone S, Mills M, Howark CJ, Solomon S (1994) Science 263:71–75
Hickson KM, Smith IWM (2001) Int J Chem Kinet 33:165–172
Urata S, Takada A, Uchimaru T, Chandra AK (2003) Chem Phys Lett 368:215–223
Singh HJ, Mishra BK (2010) J Mol Model 16:1473–1480
Singh HJ, Mishra BK (2011) J Mol Model 17:415–422
Chandra AK (2012) J Mol Model 18:4239–4247
Chen L, Kutsuna S, Tokuhashi K, Sekiya A (2004) Chem Phys Lett 400:563–568
Singh HJ, Mishra BK, Rao PK (2010) Bull Korean Chem Soc 31:3718–3722
Beach SD, Hickson KM, Smith IWM, Tuckett RP (2001) Phys Chem Chem Phys 3:3064–3069
Yang L, Liu JY, Wan SQ, Li ZS (2009) J Comput Chem 30:565–580
Blanco MB, Barnes I, Teruel MA (2010) J Phys Org Chem 23:950–954
Ninomiya Y, Kawasaki M, Guschin A, Molina LT, Molina MJ, Wallington TJ (2000) Environ Sci Technol 34:2973–2978
Dalmasso PR, Taccone RA, Nieto JD, Teruel MA, Lane SI (2006) Atmos Environ 40:7298–7303
Wingenter OW, Kubo MK, Blake NJ, Smith TW, Blake DR (1996) J Geophys Res 101:4331–4340
Sulback Andersen MP, Nielsen OJ, Wallington TJ, Hurley MD, DeMoore GW (2005) J Phys Chem A 109:3926–3934
Quan HD, Tamura M, Gao RX, Sekiya A (2003) J Fluorine Chem 120:131–134
Sekiya A, Quan HD, Tamura M, Gao RX, Murata J (2001) J Fluorine Chem 112:145–148
Chen QY, Duan JX (1993) Tetrahedron Lett 34:4241–4244
Su DB, Duan JX, Yu AJ, Chen QY (1993) J Fluorine Chem 65:11–14
Mcharek S, Sibille S, Nedelec JY, Perichon J (1991) J Organomettallic Chem 401:211–215
Mellouki A, Bras GL, Sidebottom H (2003) Chem Rev 103:5077–5096
Blanco MB, Teruel MA (2007) Chem Phys Lett 441:1–6
Blanco MB, Teruel MA (2007) Atmos Environ 41:7330–7338
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2008) Chem Phys Lett 453:18–23
Andersen VF, Berhanu TA, Nilsson EJK, Jørgensen S, Nielsen OJ, Wallington TJ, Johnson MS (2011) J Phys Chem A 115:8906–8919
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel M (2010) Environ Sci Technol 44:2354–2359
Frisch MJ et al. (2009) GAUSSIAN 09 (Revision B.01). Gaussian Inc, Wallingford
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918
Chakrabatty AK, Mishra BK, Bhattacharjee D, Deka RC (2012) Mol Phys. doi:10.1080/00268976.2012.747707
Mishra BK, Chakrabatty AK, Deka RC (2013) J Mol Model. doi:10.1007/s00894-013-1762-7
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Curtiss LA, Raghavachari K, Pople JA (1993) J Chem Phys 98:1293–1298
Kuchitsu K (1998) Structure of free polyatomic molecules basic data, 1. Springer, Berlin, p 58
Zhurko G, Zhurko D (2011) ChemCraft 1.6 Program Revision 1.6, Ivanovo, Russia
Hammond GS (1955) J Am Chem Soc 77:334–338
Troung NT, Truhlar DG (1990) J Chem Phys 93:1761–1769
Lide DR (ed) (2008–2009) CRC handbook of chemistry and physics, 89th edn. CRC, New York
Good DA, Fransisco JS (1998) J Phys Chem A 102:7143–7148
Chase MW Jr (1998) JANAF therochemical tables, 3rd edn. J Phys Chem Ref Data 9:1–1951
Pittam DA, Pilcher G (1972) J Chem Soc Faraday Trans 68:2224–2229
Wiberg KB, Crocker LS, Morgan KM (1991) J Am Chem Soc 113:3447–3450
Pilcher G, Pell AS, Coleman D (1964) J Trans Faraday Soc 60:499–505
Manion JA (2002) J Phys Chem Ref Data 31:123–172
Csontos J, Rolok Z, Das S, Kallay M (2010) J Phys Chem A 114:13093–13103
Hall HK Jr, Baldt JH (1971) J Am Chem Soc 93:140–145
Barnes DS, Pilcher G (1975) J Chem Thermodynamics 7:377–382
Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM, Churney KL, Nuttall RL (1982) J Phys Chem Ref Data 11:Suppl 2
Guthrie JP (1976) Can J Chem 54:202–209
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, New Delhi
Truhlar DG, Chuang YY (2000) J Chem Phys 112:1221–1228
Kaliginedi V, Ali MA, Rajakumar B (2012) Int J Quantum Chem 112:1066–1077
Wigner EP (1932) Z Phys Chem B19:203–216
Eckart C (1930) Phys Rev 35:1303–1309
Shavitt I (1959) J Chem Phys 31:1359–1367
Johnston HS, Rapp D (1961) J Am Chem Soc 83:1–9
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) J Phys Chem A 108:2666–2674
Kurylo MJ, Orkin VL (2003) Chem Rev 103:5049–5076
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Nature 394:353–356
Acknowledgments
BKM is thankful to University Grants Commission, New Delhi for providing UGC-Dr. D. S. Kothari Post doctoral Fellowship. Authors are also thankful to the reviewers for their constructive suggestions to improve the quality of the manuscripts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3187 kb)
Rights and permissions
About this article
Cite this article
Mishra, B.K., Chakrabartty, A.K. & Deka, R.C. Theoretical investigation of the gas-phase reactions of CF2ClC(O)OCH3 with the hydroxyl radical and the chlorine atom at 298 K. J Mol Model 19, 3263–3270 (2013). https://doi.org/10.1007/s00894-013-1865-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1865-1