Abstract
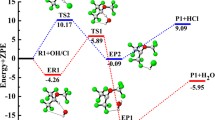
The present study deals with the decomposition of CF3OCF2O radical formed from a hydrofluoroether, CF3OCHF2 (HFE-125), in the atmosphere. The study is performed using ab initio quantum mechanical methods. Two plausible pathways of decomposition of the titled species have been considered, one involving C-O bond scission and the other occurring via F atom elimination. The geometries of the reactant, products and transition states involved in the decomposition pathways are optimized and characterized at DFT (B3LYP) level of theory using 6-311G(d,p) basis set. Single point energy calculations have been performed at G2M(CC,MP2) level of theory. Out of the two prominent decomposition channels considered, the C-O bond scission is found to be dominant involving a barrier height of 15.3 kcal mol−1 whereas the F-elimination path proceeds with a barrier of 26.1 kcal mol−1. The thermal rate constants for the above two decomposition pathways are evaluated using canonical transition state theory (CTST) and these are found to be 1.78 × 106 s−1 and 2.83 × 10−7 s−1 for C-O bond scission and F-elimination respectively at 298 K and 1 atm pressure. Transition states are searched on the potential energy surfaces involved during the decomposition channels and each of the transition states is characterized. The existence of transition states on the corresponding potential energy surface is ascertained by performing intrinsic reaction coordinate (IRC) calculation.




Similar content being viewed by others
References
Solomon S (1990) Nature (London) 6291:347–354
Molina MJ, Rowland FS (1974) Nature 249:810–814
Rowland FS, Molina MJ (1994) Chem Eng News 8:72–76
Weubbles DJ (1983) J Geophys Res 88:1433–1443
Scientific Assessment of Stratospheric Ozone (1989) Vol 11; World Meteorological Organization, Global Ozone Research and Monitoring Project Report No. 20, Geneva
Wayne RP (2001) Chemistry of atmospheres. Clarendon press, Oxford
Dekant W (1996) Environ Health Perspect 104:75–83
Hayman G, Derwent RD (1997) Environ Sci Technol 31:327–336
Tsai WT, Chen HP, Hsien WY (2002) J Loss Prev Process Ind 15:65–75
Tsai WT (2005) Chemosphere 61:1539–1547
Pinnock S, Hurly M, Shine KP, Wallington TJ, Smyth TJ (1995) J Geophys Res 100:23227–23238
International Panel on Climate Change (IPCC) (1996) Climate change 1995: the science of climate change. Cambridge University Press, New York
Tsai WT (2005) J Hazard Mater 119:69–78
Sekiya A, Misaki S (2000) J Fluorine Chem 101:215–221
Bivens DB, Minor BH (1998) Int J Refrig 21:567–576
Cooper DL, Cunningham TP, Allan NL, McCulloch A (1992) Atmos Environ 26A:1331–1334
Blowers P, Tetrault KF, Morehead YT (2008) Theor Chem Acc 119:369–381
Ravishankara RA, Turnipseed AA, Jensen NR, Barone S, Mills M, Howark CJ, Solomon S (1994) Science 263:71–75
Granier C, Shine KP, Daniel JS, Hansen JE, Lal S, Stordal F (1999) Climate effects of ozone and halocarbon changes, in scientific Assessment of Ozone Depletion: 1998 Rep. 44, Global Ozone Research and Monitoring Project. World Meteorological Organization, Geneva
Good DA, Francisco JS (1998) J Phys Chem 102:1854–1864
Sekiya A, Misaki S (1996) Chemtech 26:44–48
Smith ND (1992) EPA-60/F-92-012. Springerfield
Good DA, Mike K, Randy S, Francisco JS (1999) J Phys Chem 103:9230–9240
Murray JS, Toro-Labbe A, Clark T, Politzer P (2009) J Mol Model 15:701–706
Atkinson R (1990) Atoms Environ 24A:1–41
Hehre WJ, Radom L, PvR S, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Frisch MJ, Trucks GW, Schlegel HB et al (2003) Gaussian 03, Rev C.02. Gaussian Inc, Pittsburgh
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Gonzalez C, Schlegel HB (1990) J Chem Phys 94:5523–5527
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) J Chem Phys 94:7221–7230
Mebel AM, Morokuma K, Lin MC (1995) J Chem Phys 103:7414–7421
Curtiss LA, Redfern PC, Smith BJ, Radom L (1996) J Chem Phys 104:5148–5164
Purvis GD, Bartlett RJ (1982) J Chem Phys 76:1910–1918
Frisch A, Nielsen AB, Holder AJ (2000) GaussView reference. Gaussian Inc, Wallingford
Scott AP, Radom L (1996) J Phys Chem 100:16502–16513
Truhlar DG, Garrett BC, Klippenstein SJ (1996) J Phys Chem 100:12771–12800
Wigner EP (1932) Z Phys Chem B19:203–216
Somnitz H, Zellner R (2001) Phys Chem Chem Phys 3:2352–2364
Henon E, Bohr F, Sokolowski Gomez N, Caralp F (2003) Phys Chem Chem Phys 5:5431–5437
Acknowledgments
One of the authors (BKM) is thankful to University Grants Commission, New Delhi for providing financial assistance under its Department of Special Assistance / Basic Scientific Research Program sanctioned to the Department of Chemistry, Deendayal Upadhyay Gorakhpur University, Gorakhpur. Authors are thankful to the reviewer for making valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.J., Mishra, B.K. Ab initio studies on the decomposition kinetics of CF3OCF2O radical. J Mol Model 17, 415–422 (2011). https://doi.org/10.1007/s00894-010-0735-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0735-3