Abstract
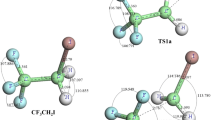
Hydrofluoroethers are being considered as potential candidates for third generation refrigerants. The present investigation involves the ab initio quantum mechanical study of the decomposition mechanism of CF3OCH2O radical formed from a hydrofluoroether, CF3OCH3 (HFE-143a) in the atmosphere. The geometries of the reactant, products and transition states involved in the decomposition pathways are optimized and characterized at the DFT (B3LYP) level of theory using 6-311G(d,p) basis set. Energy calculations have been performed at the G2(MP2) and G2M(CC,MP2) level of theory. Two prominent decomposition channels, C-O bond scission and reaction with atmospheric O2 have been considered for detailed investigation. Studies performed at the G2(MP2) level reveals that the decomposition channel involving C-O bond scission occurs with a barrier height of 23.8 kcal mol−1 whereas the oxidative pathway occurring with O2 proceeds with an energy barrier of 7.2 kcal mol−1. On the other hand the corresponding values at G2M(CC,MP2) are 24.5 and 5.9 kcal mol−1 respectively. Using canonical transition state theory (CTST) rate constants for the two pathways considered are calculated at 298 K and 1 atm pressure and found to be 5.9 × 10−6 s−1 and 2.3 × 10−5 s−1 respectively. The present study concludes that reaction with O2 is the dominant path for the consumption of CF3OCH2O in the atmosphere. Transition states are searched and characterized on the potential energy surfaces involved in both of the reaction channels. The existence of transition state on the corresponding potential energy surface is ascertained by performing intrinsic reaction coordinate (IRC) calculation.




Similar content being viewed by others
References
Solomon S (1990) Nature (London) 6291:347–354
Molina MJ, Rowland FS (1974) Nature 249:810–814
Rowland FS, Molina MJ (1994) Chem Eng News 8:72–76
Weubbles DJ (1983) J Geophys Res 88:1433–1443
Scientific Assessment of Stratospheric Ozone, Vol 11. World Meteorological Organization, Global Ozone Research and Monitoring Project Report No. 20, 1989
Wayne RP (2001) Chemistry of Atmospheres. Clarendon press, Oxford
Pinnock S, Hurly M, Shine KP, Wallington TJ, Smyth TJ (1995) J Geophys Res 100:23227–23238
International Panel on Climate Change (IPCC) (1996) Climate change 1995: the science of climate change. Cambridge University Press, New York
Tsai WT (2005) J Hazard Mater 119:69–78
Sekiya A, Misaki S (2000) J Fluorine Chem 101:215–221
Bivens DB, Minor BH (1998) Int J Refrig 21:567–576
Ravishankara RA, Turnipseed AA, Jensen NR, Barone S, Mills M, Howark CJ, Solomon S (1994) Science 263:71–75
Granier C, Shine KP, Daniel JS, Hansen JE, Lal S, Stordal F (1999) Climate effects of ozone and halocarbon changes, in scientific Assessment of Ozone Depletion: 1998 Rep. 44, Global Ozone Research and Monitoring Project. World Meteorological Organization, Geneva, Switzerland
Cooper DL, Cunningham TP, Allan NL, McCulloch A (1992) Atmos Env 26A:1331–1334
Cooper DL, Cunningham TP, Allan NL, McCulloch A (1993) Atmos Env 27A:117–119
Chen L, Kutsuna S, Tokuhashi K, Sekiya A (2006) J Phys Chem A 110:12845–12851
Urata S, Takada A, Uchimaru T, Chandra AK (2003) Chem Phys Lett 368:215–223
Chen L, Kutsuna S, Tokuhashi K, Sekiya A, Takeuchi K, Ibusuki T (2003) Int J Chem Kinet 35:239–245
Sun H, Gong H, Pan X, Hao L, Sun CC, Wang R, Huang X (2009) J Phys Chem A 113:5951–5957
Wallington TJ, Schneider WF, Sehested J, Blide M, Platz J, Nielsen OJ, Christensen LK (1997) J Phys Chem A 101:8264–8274
Atkinson R (1990) Atmos Environ 24A:1–41
Wallington TJ, Hurley MD, Fracheboud JM, Orlando JJ, Tyndall GS, Sehested J, Mogelberg TE, Nielsen OJ (1996) J Phys Chem 100:18116–18122
Christensen LK, Wallington TJ, Guschin A, Hurly MD (1999) J Phys Chem A 102:1854–1864
Good DA, Mike K, Randy S, Francisco JS (1999) J Phys Chem 103:9230–9240
Good DA, Francisco JS (1998) J Phys Chem A 102:1854–1864
Frisch MJ, Trucks GW, Schlegel HB et al. (2003) Gaussian 03, Rev C.02. Gaussian Inc, Pittsburgh, PA
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Gonzalez C, Schlegel HB (1990) J Chem Phys 94:5523–5527
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) J Chem Phys 94:7221–7230
Curtiss LA, Redfern PC, Smith BJ, Radom L (1996) J Chem Phys 104:5148–5164
Mebel AM, Morokuma K, Lin MC (1995) J Chem Phys 103:7414–7421
Frisch A, Nielsen AB, Holder AJ (2000) GaussView Reference. Gaussian Inc, Wallingford, CT
Scott AP, Radom L (1996) J Phys Chem 100:16502–16513
Truhlar DG, Garrett BC, Klippenstein SJ (1996) J Phys Chem 100:12771–12800
Wigner EP (1932) Z Phys Chem B 19:203–216
Acknowledgments
One of the authors, BKM is thankful to University Grants Commission, New Delhi for providing fellowship under its Special Assistance Program (SAP) sanctioned to the Department of Chemistry, DDU Gorakhpur University, Gorakhpur.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.J., Mishra, B.K. Ab initio studies on the reactivity of the CF3OCH2O radical: Thermal decomposition vs. reaction with O2 . J Mol Model 16, 1473–1480 (2010). https://doi.org/10.1007/s00894-010-0665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0665-0