Abstract
Background
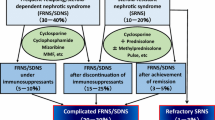
Although rituximab effectively prevents relapses of complicated frequently relapsing nephrotic syndrome (FRNS) and steroid-dependent nephrotic syndrome (SDNS), data of long-term outcomes and safety are limited.
Methods
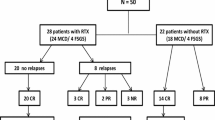
Fifty-one patients (age, 3–38 years) with childhood-onset complicated FRNS or SDNS, who received rituximab in investigator-initiated multicenter prospective trials were enrolled. Rituximab was administered at 375 mg/m2 once weekly for 4 weeks, and immunosuppressive agents were discontinued according to the study protocol. We investigated relapses, re-administration of immunosuppressive agents, additional rituximab treatment, body height, renal function, and late adverse events during the observation period.
Results
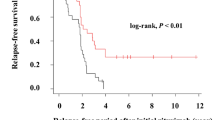
Forty-eight patients (94%) developed relapses during the observation period (median, 59 months) and the 50% relapse-free survival was 261 days. Thirty patients (59%) developed SDNS, 44 (86%) required re-administration of immunosuppressive agents, and 22 (43%) received additional rituximab treatment. All patients who were receiving immunosuppressive agents at rituximab treatment required either immunosuppressive agents or additional rituximab treatment. On the contrary, 5 of the 13 patients without immunosuppressive agents at rituximab treatment required neither immunosuppressive agents nor additional rituximab treatment and 3 of them did not develop relapse during observation period. Growth failure due to steroid toxicity did not progress and none of the patients developed chronic renal insufficiency. None of the patients suffered from rituximab-related late adverse events.
Conclusions
As most patients suffer from relapses after B-cell recovery, long-term immunosuppressive agents or additional rituximab treatment is necessary. However, some patients who can discontinue immunosuppressive agents before rituximab treatment may achieve long-term remission after rituximab treatment without immunosuppressive agents.



Similar content being viewed by others
References
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, Saito M, Yoneko M, Iijima K (2009) Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 24:1321–1328
Ishikura K, Yoshikawa N, Nakazato H, Sasaki S, Nakanishi K, Matsuyama T, Ito S, Hamasaki Y, Yata N, Ando T, Iijima K, Honda M, Japanese Study Group of Renal Disease in Children (2015) Morbidity in children with frequently relapsing nephrosis: 10-year follow-up of a randomized controlled trial. Pediatr Nephrol 30:459–468
Kamei K, Ogura M, Sato M, Sako M, Iijima K, Ito S (2016) Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol 31:89–95
Sellier-Leclerc AL, Baudouin V, Kwon T, Macher MA, Guérin V, Lapillonne H, Deschênes G, Ulinski T (2012) Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood--follow-up after CD19 recovery. Nephrol Dial Transplant 27:1083–1089
Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, Pape L, Patzer L, Billing H, Weber L, Pohl M, Rosenthal K, Rosahl A, Mueller-Wiefel DE, Dötsch J (2012) Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 27:1910–1915
Tellier S, Brochard K, Garnier A, Bandin F, Llanas B, Guigonis V, Cailliez M, Pietrement C, Dunand O, Nathanson S, Bertholet-Thomas A, Ichay L, Decramer S (2013) Long-term outcome of children treated with rituximab for idiopathic nephrotic syndrome. Pediatr Nephrol 28:911–918
Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM (2013) Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84:1025–1033
Sinha A, Bhatia D, Gulati A, Rawat M, Dinda AK, Hari P, Bagga A (2015) Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant 30:96–106
International Study of Kidney Disease in Children (1982) Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 101:514–518
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M (2014) Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol 18:626–633
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Ito S, Kamei K, Ogura M, Sato M, Fujimaru T, Ishikawa T, Udagawa T, Iijima K (2011) Maintenance therapy with mycophenolate mofetil after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:1823–1828
Fujinaga S, Someya T, Watanabe T, Ito A, Ohtomo Y, Shimizu T, Kaneko K (2013) Cyclosporine versus mycophenolate mofetil for maintenance of remission of steroid-dependent nephrotic syndrome after a single infusion of rituximab. Eur J Pediatr 172:513–518
Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW 3rd (2011) Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3:85ra46
Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group (2014) Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25:850–863
Sato M, Ito S, Ogura M, Kamei K (2014) Impact of rituximab on height and weight in children with refractory steroid-dependent nephrotic syndrome. Pediatr Nephrol 29:1373–1379
Avivi I, Stroopinsky D, Katz T (2013) Anti-CD20 monoclonal antibodies: beyond B-cells. Blood Rev 27:217–223
Stroopinsky D, Katz T, Rowe JM, Melamed D, Avivi I (2012) Rituximab-induced direct inhibition of T-cell activation. Cancer Immunol Immunother 61:1233–1241
Peuvrel L, Chiffoleau A, Quéreux G, Brocard A, Saint-Jean M, Batz A, Jolliet P, Dréno B (2013) Melanoma and rituximab: an incidental association? Dermatology 226:274–278
Velter C, Pagès C, Schneider P, Osio A, Brice P, Lebbé C (2014) Four cases of rituximab-associated melanoma. Melanoma Res 24:401–403
Aksoy S, Arslan C, Harputluoglu H, Dizdar O, Altundag K (2011) Malignancies after rituximab treatment: just coincidence or more? J BUON 16:112–115
Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, Patti C, Parvis G, Ciceri F, Gallamini A, Cortelazzo S, Zoli V, Corradini P, Carobbio A, Mulé A, Bosa M, Barbui A, Di Nicola M, Sorio M, Caracciolo D, Gianni AM, Rambaldi A (2011) Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol 29:814–824
Fleury I, Chevret S, Pfreundschuh M, Salles G, Coiffier B, van Oers MH, Gisselbrecht C, Zucca E, Herold M, Ghielmini M, Thieblemont C (2016) Rituximab and risk of second primary malignancies in patients with non-Hodgkin lymphoma: a systematic review and meta-analysis. Ann Oncol 27:390–397
Acknowledgements
The authors thank all patients and their families, in addition to the physicians who participated in the RCRNS studies and the present follow-up study. The authors thank the EDANZ group Japan for proofreading and editing the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Koichi Kamei has received lecture fees from Chugai Pharmaceutical. Kenji Ishikura has received lecture fees from Asahi Kasei Pharma, Chugai Pharmaceutical, Zenyaku Kogyo, and Novartis Pharma, and a consulting fee from Ono Pharmaceutical. Mayumi Sako has received grants from Eisai, lecture fees and/or consulting fees from Astellas Pharma and Zenyaku Kogyo. Kunihiko Aya has received lecture fees from Asahi Kasei Pharma. Kandai Nozu has received lecture fees from Chugai Pharmaceutical and Asahi Kasei Pharma. Koichi Nakanishi has received lecture fees from Otsuka Pharmaceutical and Asahi Kasei Pharma. Shuichi Ito has received lecture fees and/or grants from Chugai Pharmaceutical, Zenyaku Kogyo, Asahi Kasei Pharma, Novartis Pharma, and Astellas Pharma. Hidefumi Nakamura is an advisor to Takeda Pharmaceutical and received lecture fees from Mitsubishi Tanabe Pharma. Kazumoto Iijima has received grants from Novartis Pharma, Pfizer Japan, Kyowa Hakko Kirin, AbbVie, Daiichi Sankyo, Teijin Pharma, CSL Behring, and Astellas Pharma, lecture fees and/or consulting fees from Pfizer Japan, Asahi Kasei Pharma, Novartis Pharma, Zenyaku Kogyo, Daiichi Sankyo, Chugai Pharmaceutical, Astellas Pharma, and Takeda Pharmaceutical.
Ethics approval
The design and execution of this study were in accordance with the ethical standards of the Declaration of Helsinki. The protocol was approved by the Ethics Committee of the National Center for Child Health and Development (No. 774).
Informed consent
For this type of study, formal informed consent is not required.
Rights and permissions
About this article
Cite this article
Kamei, K., Ishikura, K., Sako, M. et al. Long-term outcome of childhood-onset complicated nephrotic syndrome after a multicenter, double-blind, randomized, placebo-controlled trial of rituximab. Pediatr Nephrol 32, 2071–2078 (2017). https://doi.org/10.1007/s00467-017-3718-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3718-0