Abstract
Background
Although studies of robotic rectal cancer surgery have demonstrated the effects of learning on operation time, comparisons have failed to demonstrate differences in clinicopathological outcomes between unadjusted learning phases. This study aimed to investigate the learning curve of robotic rectal cancer surgery for clinicopathological outcomes and compare surgical outcomes between adjusted learning phases.
Study design
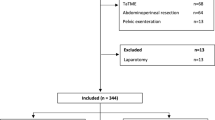
We enrolled 506 consecutive patients with rectal adenocarcinoma who underwent robotic resection by a single surgeon between 2007 and 2018. Risk-adjusted cumulative sum (RA-CUSUM) for surgical failure was used to analyze the learning curve. Surgical failure was defined as the occurrence of any of the following: conversion to open surgery, severe complications (Clavien–Dindo grade ≥ 3a), insufficient number of harvested lymph nodes (LNs), or R1 resection. Comparisons between learning phases analyzed by RA-CUSUM were performed before and after propensity score matching.
Results
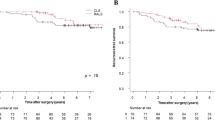
In RA-CUSUM analysis, the learning curve was divided into two learning phases: phase 1 (1st–177th cases, n = 177) and phase 2 (178th–506th cases, n = 329). Before matching, patients in phase 2 had deeper tumor invasion and higher rates of positive LNs on pretreatment images and preoperative chemoradiotherapy. After matching, phase 1 (n = 150) and phase 2 (n = 150) patients exhibited similar clinical characteristics. Phase 2 patients had lower rates of surgical failure overall and these components: conversion to open surgery, severe complications, and insufficient harvested LNs.
Conclusions
For robotic rectal cancer surgery, surgical outcomes improved after the 177th case. Further studies by other robotic surgeons are required to validate our results.


Similar content being viewed by others
References
Miyajima N, Fukunaga M, Hasegawa H, Tanaka J, Okuda J, Watanabe M (2009) Results of a multicenter study of 1057 cases of rectal cancer treated by laparoscopic surgery. Surg Endosc 23:113–118
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Jiménez-Rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-Pavón JM, Reyes-Díaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F (2016) Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 31:1807–1815
Akmal Y, Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A (2012) Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc 26:2471–2476
Park EJ, Kim CW, Cho MS, Kim DW, Min BS, Baik SH, Lee KY, Kim NK (2014) Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer? A comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine 93:e109
Jiménez-Rodríguez RM, Díaz-Pavón JM, de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28:815–821
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 242:83–91
Son GM, Kim JG, Lee JC, Suh YJ, Cho HM, Lee YS, Lee IK, Chun CS (2010) Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech 20:609–617
Kaul S, Shah NL, Menon M (2006) Learning curve using robotic surgery. Curr Urol Rep 7:125–129
Dal Moro F, Secco S, Valotto C, Artibani W, Zattoni F (2012) Specific learning curve for port placement and docking of da Vinci(®) Surgical System: one surgeon's experience in robotic-assisted radical prostatectomy. J Robot Surg 6:323–327
Hashimoto T, Yoshioka K, Gondo T, Kamoda N, Satake N, Ozu C, Horiguchi Y, Namiki K, Nakashima J, Tachibana M (2013) Learning curve and perioperative outcomes of robot-assisted radical prostatectomy in 200 initial Japanese cases by a single surgeon. J Endourol 27:1218–1223
Raimondi P, Marchegiani F, Cieri M, Cichella A, Cotellese R, Innocenti P (2018) Is right colectomy a complete learning procedure for a robotic surgical program? J Robot Surg. 12:147–155
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 28:2821–2831
Park YA, Kim JM, Kim SA, Min BS, Kim NK, Sohn SK, Lee KY (2010) Totally robotic surgery for rectal cancer: from splenic flexure to pelvic floor in one setup. Surg Endosc 24:715–720
Gouvas N, Georgiou PA, Agalianos C, Tzovaras G, Tekkis P, Xynos E (2018) Does conversion to open of laparoscopically attempted rectal cancer cases affect short- and long-term outcomes? A systematic review and meta-analysis. J Laparoendosc Adv Surg Tech 28:117–126
Duraes LC, Stocchi L, Steele SR, Kalady MF, Church JM, Gorgun E, Liska D, Kessler H, Lavryk OA, Delaney CP (2018) The relationship between Clavien-Dindo morbidity classification and oncologic outcomes after colorectal cancer resection. Ann Surg Oncol 25:188–196
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26:303–312
Xiao WW, Zhang LN, You KY, Huang R, Yu X, Chang H, Ding PR, Gao YH (2018) The total number of lymph nodes harvested from pathological T3N0 rectal cancer patients: prognostic significance and potential indication for postoperative radiotherapy. J Cancer Res Ther 14:S288–S294
Han J, Noh GT, Yeo SA, Cheong C, Cho MS, Hur H, Min BS, Lee KY, Kim NK (2016) The number of retrieved lymph nodes needed for accurate staging differs based on the presence of preoperative chemoradiation for rectal cancer. Medicine 95:e4891
Kim CH, Kim HJ, Huh JW, Kim YJ, Kim HR (2014) Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol 110:989–996
Li J, Wang Y, Liu D, Zhou H, Mou T, Li G, Deng H (2018) Multidimensional analyses of the learning curve for single-incision plus one port laparoscopic surgery for sigmoid colon and upper rectal cancer. J Surg Oncol 117:1386–1393
Orsenigo E, Gasparini G, Carlucci M (2019) Clinicopathological factors influencing lymph node yield in colorectal cancer: a retrospective study. Gastroenterol Res Pract 2019:5197914. https://doi.org/10.1155/2019/5197914
Betge J, Harbaum L, Pollheimer MJ, Lindtner RA, Kornprat P, Ebert MP, Langner C (2017) Lymph node retrieval in colorectal cancer: determining factors and prognostic significance. Int J Colorectal Dis 32:991–998
Morcos B, Baker B, Al Masri M, Haddad H, Hashem S (2010) Lymph node yield in rectal cancer surgery: effect of preoperative chemoradiotherapy. Eur J Surg Oncol 36:345–349
Miller ED, Robb BW, Cummings OW, Johnstone PA (2012) The effects of preoperative chemoradiotherapy on lymph node sampling in rectal cancer. Dis Colon Rectum 55:1002–1007
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J (2016) Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC Multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol 34:3300–3307
Zhong QH, Wu PH, Qin QY, Kuang YY, Ma TH, Wang HM, Zhu YX, Chen DC, Wang JP, Wang L (2017) Pathological insights of radiotherapy-related damage to surgical margin after preoperative radiotherapy in patients with rectal cancer. Zhonghua Wai Ke Za Zhi 55:507–514
Qin Q, Zhu Y, Wu P, Fan X, Huang Y, Huang B, Wang J, Wang L (2019) Radiation-induced injury on surgical margins: a clue to anastomotic leakage after rectal-cancer resection with neoadjuvant chemoradiotherapy? Gastroenterol Rep 7:98–106
Bae SU, Baek SJ, Hur H, Baik SH, Kim NK, Min BS (2013) Intraoperative near infrared fluorescence imaging in robotic low anterior resection: three case reports. Yonsei Med J 54:1066–1069
Philippou P, Waine E, Rowe E (2012) Robot-assisted laparoscopic prostatectomy versus open: comparison of the learning curve of a single surgeon. J Endourol 26:1002–1008
Chen W, Li Q, Fan Y, Li D, Jiang L, Qiu P, Tang L (2016) Factors predicting difficulty of laparoscopic low anterior resection for rectal cancer with total mesorectal excision and double stapling technique. PLoS ONE 11:e0151773. https://doi.org/10.1371/journal.pone.0151773
Escal L, Nougaret S, Guiu B, Bertrand MM, de Forges H, Tetreau R, Thézenas S, Rouanet P (2018) MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg 105:140–146
Duchalais E, Machairas N, Kelley SR, Landmann RG, Merchea A, Colibaseanu DT, Mathis KL, Dozois EJ, Larson DW (2018) Does prolonged operative time impact postoperative morbidity in patients undergoing robotic-assisted rectal resection for cancer? Surg Endosc 32:3659–3666
Foo CC, Law WL (2016) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40:456–462
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29:1679–1685
Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, Sender Liberman A, Min BS (2015) Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 29:558–568
Kim HJ, Choi GS, Park JS, Park SY (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon's experience. Dis Colon Rectum 57:1066–1074
Sng KK, Hara M, Shin JW, Yoo BE, Yang KS, Kim SH (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27:3297–3307
Acknowledgments
The authors thank Youn Ho Roh, a biostatistician, for methodological advice regarding the RA-CUSUM analysis used in this study.
Funding
No financial support.
Author information
Authors and Affiliations
Contributions
JML, NKK: Conception and design. JML: Collection and assembly of data. JML, SYY, YDH, MSC, HH, BSM, KYL: Data analysis and interpretation. All authors: Manuscript writing. All authors: Final approval of manuscript.
Corresponding author
Ethics declarations
Discloures
Dr. Jong Min Lee, Dr. Seung Yoon Yang, Dr. Yoon Dae Han, Dr. Min Soo Cho, Dr. Hyuk Hur, Dr. Byung Soh Min, Dr. Kang Young Lee and Dr. Nam Kyu Kim have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
464_2020_7445_MOESM1_ESM.tif
Supplementary file1 Supplementary Figure 1. Cumulative sum (CUSUM) for operation time. CUSUM peaked at the 133th case.(TIF 4332 kb)
Rights and permissions
About this article
Cite this article
Lee, J.M., Yang, S.Y., Han, Y.D. et al. Can better surgical outcomes be obtained in the learning process of robotic rectal cancer surgery? A propensity score-matched comparison between learning phases. Surg Endosc 35, 770–778 (2021). https://doi.org/10.1007/s00464-020-07445-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07445-3