Abstract
Although the short-term outcomes of robot-assisted laparoscopic surgery (RALS) for rectal cancer are well known, the long-term oncologic outcomes of RALS compared with those of conventional laparoscopic surgery (CLS) are not clear. This study aimed to compare the long-term outcomes of RALS and CLS for rectal cancer using propensity score matching. This retrospective study included 185 patients with stage I–III rectal cancer who underwent radical surgery at our institute between 2010 and 2019. Propensity score analyses were performed with 3-year overall survival (OS) and relapse-free survival (RFS) as the primary endpoints. After case matching, the 3-year OS and 3-year RFS rates were 86.5% and 77.9% in the CLS group and 98.4% and 88.5% in the RALS group, respectively. Although there were no significant differences in OS (p = 0.195) or RFS (p = 0.518) between the groups, the RALS group had slightly better OS and RFS rates. 3-year cumulative (Cum) local recurrence (LR) and 3-year Cum distant metastasis (DM) were 9.7% and 8.7% in the CLS group and 4.5% and 10.8% in the RALS group, respectively. There were no significant differences in Cum-LR (p = 0.225) or Cum-DM (p = 0.318) between the groups. RALS is a reasonable surgical treatment option for patients with rectal cancer, with long-term outcomes similar to those of CLS in such patients.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Of the various types of surgery for rectal cancer, minimally invasive surgery (MIS) is increasingly being performed for patients with this condition. Compared to open surgery (OS), MIS for rectal cancer has many advantages owing to the introduction of advanced instruments and evolution of surgical techniques. Evaluation of conventional laparoscopic surgery (CLS) for rectal cancer in randomized clinical trials [1, 2] revealed that it had similar or better short-term and similar long-term oncological outcomes compared to those of OS [3, 4].
One recent study examined the short-term results for rectal cancer of the latest approach, robot-assisted laparoscopic surgery (RALS). The ROLARR randomized clinical trial compared RALS with CLS for rectal cancer and tried to evaluate procedural superiority in terms of conversion rates; however, the results were not definitive [5]. Nonetheless, favorable safety and technical feasibility-related outcomes of RALS for rectal cancer have been reported in several retrospective case–control studies [6,7,8,9,10] and small randomized clinical trials [11,12,13]. However, evidence regarding the long-term outcomes of RALS remains scarce [14,15,16]. Furthermore, only one study has reported long-term RALS results using propensity score matching [17]. Our hospital was one of the first to introduce robotic surgical systems in Japan.
Propensity score analysis is used to minimize selection bias in retrospective studies and increase the strength of the evidence. Therefore, this study used propensity scores to compare the long-term results of RALS and CLS for rectal cancer.
Methods
Patients
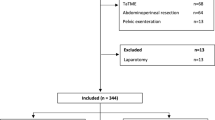
In total, 311 patients who underwent radical surgery for stage I–III rectal cancer at our institute between 2010 and 2019 were retrospectively reviewed. Of these patients, we excluded those for whom we did not have sufficient data. Finally, we divided the remaining 185 patients into the CLS and RALS groups according to the surgical procedure they underwent. Based on the Union for International Cancer Control (UICC) Tumor-Node-Metastasis (TNM) Classification, 8th edition, 23 patients who were diagnosed with cN-positive lower rectal cancer underwent surgery with lateral lymph node dissection (LLND) after receiving neoadjuvant chemotherapy (NAC). This study was reviewed and approved by the institutional review board of our institute, and the requirement for written informed consent was waived due to the retrospective design.
Surgical procedures
All the patients underwent gross curative RALS or CLS with lymph node dissection. Using the Da Vinci Si or Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) as a six- or five-port system, two certified surgeons performed RALS. Five ports were generally placed when using the Xi Surgical System; six ports were placed in some cases when suction was needed for excessive fluid, such as for patients who underwent NAC. Six ports are generally placed when using the Si Surgical System. The patients were placed in a lithotomy position with the head down at 15° and the right side down at 15°. All procedures were performed in the colonic or pelvic phases. Inferior mesenteric artery, vein ligation, and left-sigmoid mesocolon mobilization were performed in the colonic phase, while pelvic dissection using total mesorectal excision (TME) or tumor-specific mesorectal excision (TSME) was performed during the pelvic phase. During TSME for tumors of the upper or mid-rectum, the distal rectum was divided > 3 cm below the lower border of the tumor. In contrast, during TME for tumors of the lower rectum, the distal rectum was divided > 2 cm below the lower border of the tumor. The distal rectum was intracorporeally divided using a linear stapler. Bowel continuity was restored using the intracorporeal double-stapling technique with a circular stapler or transanal hand-sewn suture in cases of intersphincteric resection (ISR). En bloc regional lymphadenectomy was performed for all patients who underwent standard curative resection. When the short diameter of the mesenteric or lateral lymph node was > 7 mm on preoperative magnetic resonance imaging (MRI), LLND was performed after NAC. LLND was performed around the common and internal iliac vessels, obturator space, and in the fat tissue outside the pelvic plexus while preserving all autonomic nerves. Diverting ileostomy was performed, if necessary, during low anterior resection and intersphincteric resection.
Follow-up
The median follow-up period was 69.9 months (range, 1.1–147.9 months). Patients with stage I–III tumors were followed-up for five years post-operatively. We measured tumor markers every three months for the first two years; thereafter, we measured the tumor markers and performed computed tomography scans every six months for the next three years. If recurrence was suspected, MRI and/or positron emission tomography was used to confirm metastasis. Surgery was considered for patients with a good performance status and resectable recurrence. When the first recurrence occurred, the recurrence site and date were recorded. Local recurrence (LR) was defined as any histological or clinical evidence of tumor regrowth near the primary site. Tumor recurrence at distant sites, such as the liver or lungs, was recorded as distant metastasis (DM). Postoperative urinary function and sexual function, were assessed using the International Prostate Symptom Score (IPSS),
Statistical analysis
The primary outcomes were the 3-year OS and 3-year relapse-free survival (RFS). We defined OS as the duration between the date of surgery and date of death or end of the observation period. Patients who were alive at the end of the follow-up period were excluded. We defined RFS as the duration between the date of surgery and the date of recurrence or death from the underlying disease. Patients who died from causes other than colorectal cancer were also excluded. Survival outcomes were examined using the Kaplan–Meier method and compared using the log-rank test. Propensity score analyses were performed to adjust for the heterogeneity between the CLS and RALS groups. Propensity scores predicting the probability of receiving CLS or RALS were generated by multivariate logistic regression with the following fourteen covariates: age, sex (female/male), body mass index (BMI), tumor location (lower rectum [0–5 cm]/middle rectum [6–10 cm]/upper rectum [> 10 cm]), neoadjuvant therapy, LLND, pathological T-stage, pathological lymph node metastasis (positive/negative), lymphatic invasion (positive/negative), venous invasion (positive/negative), differentiation (well/others), tumor size, radial margin (positive/negative), anastomotic leakage (yes/no), and postoperative complications (Clavien–Dindo class > 1). Each patient was assigned an estimated propensity score that represented the predicted probability of receiving CLS or RALS. We performed propensity score matching to pair the patients based on similarities in their characteristics. Each patient who underwent CLS was matched to a patient who underwent RALS and had a similar propensity score on the logit scale, with a caliper of 0.2. All statistical analyses were performed using SPSS version 25 (IBM® SPSS® Statistics 25.0 Windows® client version; IBM, Chicago, IL, USA). The level of statistical significance was set at p < 0.05.
Results
Short-term outcomes
There were 108 and 77 patients in the CLS and RALS groups, respectively. The matched cohort comprised 76 pairs of patients. The characteristics of the patients in the two groups are shown in Table 1.. Patients in the RALS group were more likely to have a longer operative time (p = 0.003), shorter length of hospital stay (p = 0.022), and IPS (p < 0.001) before case matching. No significant differences were observed in covariates or other outcomes. After case matching, the clinical outcomes in terms of the covariates examined were similar between the CLS and RALS groups. Patients in the RALS group were more likely to have a longer operative time (p < 0.001) and a lower IPSS (p < 0.001) after case matching.
Overall and relapse-free survival
Before case matching, the 3-year OS and 3-year RFS rates were 86.7% and 76.8% in the CLS group and 98.4% and 88.6% in the RALS group, respectively. There were no significant differences in OS (p = 0.14) or RFS (p = 0.08) between the groups.
After case matching, the 3-year OS and 3-year RFS rates were 86.5% and 77.9% in the CLS group and 98.4% and 88.5% in the RALS group, respectively. Although there were no significant differences in OS (p = 0.19) or RFS (p = 0.52) between the groups, the RALS group had slightly better OS and RFS rates (Fig. 1). The OS and RFS at each stage in the matched cohort are shown in Fig. 2. The OS and RFS at pathological T1/2 and T3/4 in the matched cohort are shown in Fig. 3.
Overall survival and recurrence-free survival rates at each stage in the matched cohort. A Overall survival rates and recurrence-free survival rates in stage I rectal cancer, B Overall survival rates and recurrence-free survival rates in stage II rectal cancer, C Overall survival rates and recurrence-free survival rates in stage III rectal cancer
Patterns of recurrence
Before case matching, the 3-year cumulative (Cum) LR and DM rates were 11.7% and 13.8% in the CLS group and 4.4% and 10.7% in the RALS group, respectively. There were no significant differences in Cum-LR (p = 0.08) or Cum-DM (p = 0.87) between the groups. After case matching, the 3-year Cum-LR and Cum-DM were 9.7% and 8.7% in the CLS group and 4.5% and 10.8% in the RALS group, respectively. There were no significant differences in Cum-LR (p = 0.23) or Cum-DM (p = 0.32) between the groups (Fig. 4).
Discussion
Colorectal cancer is the second leading cause of cancer-related death in Japan. Surgical resection of colorectal cancer is the only curative treatment that is now increasingly performed using the robotic approach. However, its long-term oncological safety has not been fully verified. The present study aimed to retrospectively clarify the short- and long-term outcomes of RALS in consecutive patients with rectal cancer. In a minimal bias analysis with propensity score matching, the primary endpoint, 3-year RFS, was comparable to that reported previously in the RALS group [18, 19]. CLS and RALS showed comparable long-term oncologic outcomes in the present study. Furthermore, RLAS had better outcomes although the difference was not statistically significant. It is well known that the relapse of colon cancer is time-associated, with nearly 70% of recurrences occurring within the first two years after initial curative surgery [20]. Our primary endpoint was 3-year RFS, and a close look at the RFS survival curves in the matched cohort showed a large gap in survival rates between RALS group and CLS group around 3 years post-operatively. In the stage-by-stage analysis, the long-term results in the present study were comparable to those of known reports [15, 21]. Regardless of stage, there was no statistically significant difference in long-term oncologic outcomes between the two groups, but RALS tended to have better long-term results, suggesting the long-term oncological advantage of RALS over CLS. Furthermore, T4 rectal cancer is a challenging condition due to the highly invasive nature of the tumor, which can compromise curative resection. However, RALS for T4 rectal cancer has been reported to be associated with a significantly lower conversion rate, shorter hospital stay, and better long-term oncological outcomes than CLS [22]. In the present study, even in deeper tumor such as pathological T3/4, RLAS tended to have similar or better OS and RFS compared to CLS.
For rectal cancer, we focused on LR control by introducing neoadjuvant therapy and lateral lymph node dissection (LLND). However, DM occurs in approximately 30% of patients at 5 years after curative surgery [23]. In the present study, RALS did not contribute significantly to DM control; however, our findings suggest that it is associated with better local control than CLS. These long-term outcomes were similar to those reported previously [14, 20]. Concerns regarding laparoscopic rectal cancer surgery include technical limitations, such as a limited range of instrument movement and assistant-dependent camera control and traction, which are aggravated in the narrow surgical space during TME. However, currently available robotic surgical systems provide advanced technology and are used as an optional approach for TME. Robotic techniques have improved ergonomics, use articulated instruments, and provide a stable 3-dimensional view along with enhanced dexterity with tremor filtration and motion scaling. The superiority of local control by RALS, along with the development of distant control treatment with total neoadjuvant therapy, suggests the potential for improved outcomes in rectal cancer.
The short-term outcomes were not significantly different between the two groups after case matching. In the ROLARR randomized clinical trial, there was no significant difference in urinary dysfunction between the two groups. However, in the present study, IPSS scores were significantly better in the RALS group. These results are consistent with those reported in the literature [23, 24]. RALS with TME is associated with earlier recovery of normal urinary and sexual function than is CLS [6]. Further, the rate of urinary retention is also reportedly lower with RALS than with CLS or OS [7, 10]. A clear surgical field, in addition to high-quality three-dimensional imaging, may facilitate a decrease in the incidence of nerve injury, such as injury of the pelvic splanchnic nerves and inferior hypogastric plexus.
This study had some limitations. First, this was a single-center retrospective study. Second, although we performed propensity analyses to address this limitation, the study remains limited by the richness of the available data, and it might not be possible to make inferences due to hidden confounders. Fourteen covariates were included in the model used to calculate the propensity scores, but other covariates, such as symptoms, nutritional status, and social activity, were not included. Third, the sample size is relatively small. A prospective study is needed to adjust for other confounders and examine prognostic outcomes in our institute in future.
In conclusion, RALS is a reasonable option for the treatment of rectal cancer with long-term outcomes similar to those of CLS.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645. https://doi.org/10.1016/s1470-2045(10)70131-5
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218. https://doi.org/10.1016/s1470-2045(13)70016-0
Park JW, Kang SB, Hao J, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim JS, Lee HS, Kim JH, Jeong SY, Oh JH (2021) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol Hepatol 6:569–577. https://doi.org/10.1016/s2468-1253(21)00094-7
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332. https://doi.org/10.1056/NEJMoa1414882
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the rolarr randomized clinical trial. JAMA 318:1569–1580. https://doi.org/10.1001/jama.2017.7219
Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH (2012) A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 19:2485–2493. https://doi.org/10.1245/s10434-012-2262-1
Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H (2016) Robotic-assisted laparoscopic versus open lateral lymph node dissection for advanced lower rectal cancer. Surg Endosc 30:721–728. https://doi.org/10.1007/s00464-015-4266-y
Matsuyama T, Endo H, Yamamoto H, Takemasa I, Uehara K, Hanai T, Miyata H, Kimura T, Hasegawa H, Kakeji Y, Inomata M, Kitagawa Y, Kinugasa Y (2021) Outcomes of robot-assisted versus conventional laparoscopic low anterior resection in patients with rectal cancer: propensity-matched analysis of the national clinical database in Japan. BJS Open. https://doi.org/10.1093/bjsopen/zrab083
Kim HJ, Choi GS, Park JS, Park SY, Lee HJ, Woo IT, Park IK (2018) Selective lateral pelvic lymph node dissection: a comparative study of the robotic versus laparoscopic approach. Surg Endosc 32:2466–2473. https://doi.org/10.1007/s00464-017-5948-4
Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H, Yamakawa Y (2016) Robotic-assisted vs. conventional laparoscopic surgery for rectal cancer: short-term outcomes at a single center. Surg Today 46:957–962. https://doi.org/10.1007/s00595-015-1266-4
Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, Chi HS, Cho CH (2008) Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc 22:1601–1608. https://doi.org/10.1007/s00464-008-9752-z
Debakey Y, Zaghloul A, Farag A, Mahmoud A, Elattar I (2018) Robotic-assisted versus conventional laparoscopic approach for rectal cancer surgery, First Egyptian Academic Center Experience. RCT Minim Invasive Surg 2018:5836562. https://doi.org/10.1155/2018/5836562
Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH (2018) Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg 267:243–251. https://doi.org/10.1097/sla.0000000000002321
Park EJ, Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 261:129–137. https://doi.org/10.1097/sla.0000000000000613
Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furuatni A, Manabe S, Yamaoka Y, Hino H (2018) Short- and long-term outcomes of robotic-assisted laparoscopic surgery for rectal cancer: results of a single high-volume center in Japan. Int J Colorectal Dis 33:1755–1762. https://doi.org/10.1007/s00384-018-3153-0
Katsuno H, Hanai T, Masumori K, Koide Y, Matsuoka H, Tajima Y, Endo T, Mizuno M, Chong Y, Maeda K, Uyama I (2020) Short- and long-term outcomes of robotic surgery for rectal cancer: a single-center retrospective cohort study. Surg Today 50:240–247. https://doi.org/10.1007/s00595-019-01874-x
Kim J, Baek SJ, Kang DW, Roh YE, Lee JW, Kwak HD, Kwak JM, Kim SH (2017) Robotic resection is a good prognostic factor in rectal cancer compared with laparoscopic resection: long-term survival analysis using propensity score matching. Dis Colon Rectum 60:266–273. https://doi.org/10.1097/dcr.0000000000000770
Baik SH, Kim NK, Lim DR, Hur H, Min BS, Lee KY (2013) Oncologic outcomes and perioperative clinicopathologic results after robot-assisted tumor-specific mesorectal excision for rectal cancer. Ann Surg Oncol 20:2625–2632. https://doi.org/10.1245/s10434-013-2895-8
Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine 94:e522. https://doi.org/10.1097/md.0000000000000522
Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ, Francini G, Grothey A, O’Connell M, Catalano PJ, Blanke CD, Kerr D, Green E, Wolmark N, Andre T, Goldberg RM, De Gramont A (2005) Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clinical Oncol Off J Am Soc Clin Oncol 23:8664–8670. https://doi.org/10.1200/jco.2005.01.6071
Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16:200–207. https://doi.org/10.1016/s1470-2045(14)71199-4
Yamaoka Y, Kagawa H, Shiomi A, Yamakawa Y, Hino H, Manabe S, Kinugasa Y (2021) Robotic-assisted surgery may be a useful approach to protect urinary function in the modern era of diverse surgical approaches for rectal cancer. Surg Endosc 35:1317–1323. https://doi.org/10.1007/s00464-020-07509-4
Lei Y, Jiang J, Zhu S, Yi B, Li J (2022) Comparison of the short-term efficacy of two types of robotic total mesorectal excision for rectal cancer. Tech Coloproctol 26:19–28. https://doi.org/10.1007/s10151-021-02546-0
Lei X, Yang L, Huang Z, Shi H, Zhou Z, Tang C, Li T (2021) No beneficial effect on survival but a decrease in postoperative complications in patients with rectal cancer undergoing robotic surgery: a retrospective cohort study. BMC Surg 21:355. https://doi.org/10.1186/s12893-021-01309-w
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Junichi Mazaki wrote the manuscript, and Yu Kuboyama, Tago Tomoya, Kenta Kasahara, Ryutaro Udo, Tesshi Yamada, and Tetsuo Ishizaki acquired data for the study. All authors have reviewed the manuscript. Yuichi Nagakawa drafted the manuscript and revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Informed consent for participants: Waiver of consent approved by ethics committee.
Ethical approval
This study was reviewed and approved by the institutional review board of our institute. (T2019-0054).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazaki, J., Ishizaki, T., Kuboyama, Y. et al. Long-term outcomes of robot-assisted laparoscopic surgery versus conventional laparoscopic surgery for rectal cancer: single-center, retrospective, propensity score analyses. J Robotic Surg 18, 157 (2024). https://doi.org/10.1007/s11701-024-01894-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-024-01894-x