Abstract
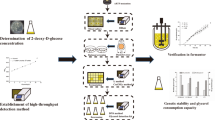
A rational high-throughput screening can significantly improve the efficiency of strain screening with high performance. In this study, based on the addition reaction of unsaturated fatty acids in the sophorolipids (SLs) and I2 molecules, a simple and rapid high-throughput detection method for SLs was established which demonstrated a correlation coefficient (R2) of 0.9106 with high-performance liquid chromatography (HPLC) method. Moreover, chlorpromazine, as a rational selecting pressure for enrichment of mutants with high cytochrome P450 enzyme activity, which was a key enzyme in the synthesis of SLs, was introduced into the high-throughput screening model. Consequently, with the aid of this effective screening system, a high-yielding mutant designated as Candida bombicola F6.5 was successfully screened out from 1500 single colonies, which presented improvements of 40.3% and 11.4% on SLs titer and yield, respectively, compared to the parent strain in a 1 L bioreactor.
Highlights
-
A simple and rapid high-throughput SLs-detecting method was established.
-
The chlorpromazine as a rational selecting pressure was introduced.
-
A high SLs-producing mutant was obtained by high-throughput screening technology.
-
40.3% and 11.4% improvements in SLs titer and yield were achieved.








Similar content being viewed by others
References
Sun XX, Choi JK, Kim EK (2004) A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J Exp Mar Biol Ecol 304(1):35–49
Kim K (2002) Characteristics of sophorolipid as an antimicrobial agent. J Microbiol Biotechnol 12(2):235–241
Kang SW, Kim YB, Shin JD, Kim EK (2010) Enhanced biodegradation of hydrocarbons in soil by microbial biosurfactant, sophorolipid. Appl Biochem Biotechnol 160(3):780–790
Oliveira MRD, Magri A, Baldo C, Camilios-Neto D, Minucelli T, Antonia M (2015) Review: sophorolipids a promising biosurfactant and it’s applications. Int J Adv Biotechnol Res 6:161–174
Rienzo MADD, Banat IM, Dolman B, Winterburn J, Martin PJ (2015) Sophorolipid biosurfactants: possible uses as antibacterial and antibiofilm agent. New Biotechnol 32(6):720–726
Zhang X, Zhang XF, Li HP, Wang LY, Zhang C, Xing XH, Bao CY (2014) Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl Microbiol Biotechnol 98(12):5387–5396
Jin Z, Lei Y, Lin J, Cen P (2006) Improvement of pristinamycin-producing Streptomyces pristinaespiralis by rational screening. World J Microbiol Biotechnol 22(2):129–134
Ribeiro IA, Bronze MR, Castro MF, Ribeiro MHL (2012) Optimization and correlation of HPLC-ELSD and HPLC–MS/MS methods for identification and characterization of sophorolipids. J Chromatogr B 899(11):72–80
Kim Y-B, Yun HS, Kim E-K (2009) Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture. Bioresour Technol 100(23):6028–6032
Van Bogaert I, Fleurackers S, Van Kerrebroeck S, Develter D, Soetaert W (2011) Production of new-to-nature sophorolipids by cultivating the yeast Candida bombicola on unconventional hydrophobic substrates. Biotechnol Bioeng 108(4):734–741
Ham B, Shelton R, Butler B, Thionville P (1998) Calculating the iodine value for marine oils from fatty acid profiles. J Am Oil Chem Soc 75(10):1445–1446
Zhong GQ (2004) Study on determination method of Iodine value of oil and fat. Sci Technol Cereals Oils Foods 12(1):29–30
Awtrey AD, Connick RE (1951) The Absorption Spectra of I2, I3-, I-, IO3- S4O6= and S2O3=. Heat of the reaction I3- = I2 + I. J Am Chem Soc 73(4):1842–1843
Morrison M, Bayse GS, Michaels AW (1971) Determination of spectral properties of aqueous I2 and I3 and the equilibrium constant. Anal Biochem 42(1):195–201
Guo T, Tang Y, Xi Y, He A, Sun B, Wu H, Liang D, Jiang M, Ouyang P (2011) Clostridium beijerinckii mutant obtained by atmospheric pressure glow discharge producing high proportions of butanol and solvent yields. Biotech Lett 33(12):2379–2383
Vamecq J (1987) Chlorpromazine and carnitine-dependency of rat liver peroxisomal beta-oxidation of long-chain fatty acids. Biochem J 241(3):783–791
Jiao P, Ma S, Huang Y, Hu P, Cao Z (2000) Two-step enrichment and double-time replica plating technology for Candida tropicalis mutant selection. J Tsinghua Univ 40(6):22–25
Zhang Y, Jia D, Sun W, Yang X, Zhang C, Zhao F, Lu W (2017) Semicontinuous sophorolipid fermentation using a novel bioreactor with dual ventilation pipes and dual sieve-plates coupled with a novel separation system. Microb Biotechnol 11(3):455–464
Acknowledgements
This work was financially supported by the National Key Research and Development Program (2017YFB0309302), the Major State Basic Research Development Program of China (973 Program, No. 2012CB721006) and the Fundamental Research Funds for the Central Universities (WF1814032, 22221817014, 22221818014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, G., Tian, X., Lin, Y. et al. Rational high-throughput system for screening of high sophorolipids-producing strains of Candida bombicola. Bioprocess Biosyst Eng 42, 575–582 (2019). https://doi.org/10.1007/s00449-018-02062-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-02062-w