Abstract
Purpose
To investigate the clinical factors that could be used predict the number of transferable blastocysts in preimplantation genetic testing (PGT) cycles based on next-generation sequencing (NGS) and formed form a mathematical model to predict the chance likelihood of obtaining one transferable blastocyst, which is helpful for genetic counseling.
Methods
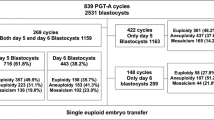
This retrospective study enrolled couples undergoing PGT cycles for chromosomal structural rearrangement (PGT-SR, n = 363, 202 with reciprocal translocation carriers, 131 with Robertsonian translocation carriers, 30 with inversion carriers), monogenic diseases (PGT-M, n = 47), and for Aneuploidies (PGT-A, n = 132) from January 2015 to October 2018. Stepwise multiple linear regression analysis was used to identify the factors relevant for obtaining at least one transferable blastocyst. The factors that predict the number of biopsied blastocysts were further analyzed.
Results
The transferable blastocyst rates were 29.94, 41.99, 49.09, 41.42, and 44.37% in the reciprocal translocation carrier, Robertsonian translocation carrier, inversion carrier, PGT-M, and PGT-A cycles, respectively. The number of transferable blastocysts in these cycles were 0.3004 × the number of biopsied blastocysts (NBB) − 0.0031, 0.4063 × NBB + 0.0460, 0.5762 × NBB − 0.3128, 0.3611 × NBB + 0.1910, and 0.4831 × NBB − 0.0970, respectively. Furthermore, the number of MII oocytes and female age were clinical predictors of NBB in reciprocal translocation and PGT-A couples, while the number of MII oocytes was the only clinical predictor in Robertsonian translocation carriers, inversion carriers, and PGT-M couples.
Conclusions
The number of biopsied blastocysts was the only clinical predictor of the ability to obtain a transferable blastocyst in PGT cycles; therefore, for clinical practice, theoretically the minimum numbers of biopsied blastocysts is 4 in reciprocal translocation carrier and 3 in couples undergoing PGT for other reasons. The number of MII oocytes and female age were clinical predictors of the number of biopsied blastocysts. With the mathematical models in our study as a reference, in clinical practice, clinicians will be able to conduct a more targeted genetic consultation for different kinds of PGT patients.

Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen H, Chen S, Ma G, Hsieh S, Tsai H, Yang Y et al (2018) Preimplantation genetic diagnosis and screening: Current status and future challenges. J Formos Med Assoc 117(2):94–100. https://doi.org/10.1016/j.jfma.2017.08.006
Hens K, Bonduelle M, de Die-Smulders C, Liebaers I (2019) Blurring boundaries. Interviews with PGT couples about comprehensive chromosome screening. Eur J Med Genet 62(12):103604. https://doi.org/10.1016/j.ejmg.2018.12.009
Brezina PR, Kutteh WH (2015) Clinical applications of preimplantation genetic testing. BMJ 350:g7611. https://doi.org/10.1136/bmj.g7611
Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W et al (2018) Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur J Hum Genet 26(1):12–33. https://doi.org/10.1038/s41431-017-0016-z
EPCS Committee, Carvalho F, Coonen E, Goossens V, Kokkali G, Rubio C et al (2020) ESHRE PGT consortium good practice recommendations for the organisation of PGT. Hum Reprod Open 2020(3):hoaa021. https://doi.org/10.1093/hropen/hoaa021
Cimadomo D, Capalbo A, Ubaldi FM, Scarica C, Palagiano A, Canipari R et al (2016) The impact of biopsy on human embryo developmental potential during preimplantation genetic diagnosis. Biomed Res Int 2016:7193075. https://doi.org/10.1155/2016/7193075
Zhang W, Liu Y, Wang L, Wang H, Ma M, Xu M et al (2016) Clinical application of next-generation sequencing in preimplantation genetic diagnosis cycles for Robertsonian and reciprocal translocations. J Assist Reprod Genet 33(7):899–906. https://doi.org/10.1007/s10815-016-0724-2
Wang L, Cram DS, Shen J, Wang X, Zhang J, Song Z et al (2014) Validation of copy number variation sequencing for detecting chromosome imbalances in human preimplantation embryos. Biol Reprod 91(2):37. https://doi.org/10.1095/biolreprod.114.120576
Xie Y, Xu Y, Wang J, Miao B, Zeng Y, Ding C et al (2018) Preliminary analysis of numerical chromosome abnormalities in reciprocal and Robertsonian translocation preimplantation genetic diagnosis cases with 24-chromosomal analysis with an aCGH/SNP microarray. J Assist Reprod Genet 35(1):177–186. https://doi.org/10.1007/s10815-017-1045-9
Mikhaail-Philips MM, Ko E, Chernos J, Greene C, Rademaker A, Martin RH (2004) Analysis of chromosome segregation in sperm from a chromosome 2 inversion heterozygote and assessment of an interchromosomal effect. Am J Med Genet A 127A(2):139–143. https://doi.org/10.1002/ajmg.a.20693
Hu X, Wang J, Li Y, Wang Y, Ding C, Zeng Y et al (2015) Clinical considerations of preimplantation genetic diagnosis for monogenic diseases. PLoS ONE 10(9):e0139613. https://doi.org/10.1371/journal.pone.0139613
Munné S (2018) Status of preimplantation genetic testing and embryo selection. Reprod Biomed Online 37(4):393–396. https://doi.org/10.1016/j.rbmo.2018.08.001
Kort DH, Chia G, Treff NR, Tanaka AJ, Xing T, Vensand LB et al (2016) Human embryos commonly form abnormal nuclei during development: a mechanism of DNA damage, embryonic aneuploidy, and developmental arrest. Hum Reprod 31(2):312–323. https://doi.org/10.1093/humrep/dev281
Cai Y, Ding M, Lin F, Diao Z, Zhang N, Sun H et al (2019) Evaluation of preimplantation genetic testing based on next-generation sequencing for balanced reciprocal translocation carriers. Reprod Biomed Online 38(5):669–975. https://doi.org/10.1016/j.rbmo.2018.12.043
Chen L, Diao Z, Xu Z, Zhou J, Wang W, Li J et al (2016) The clinical application of preimplantation genetic diagnosis for the patient affected by congenital contractural arachnodactyly and spinal and bulbar muscular atrophy. J Assist Reprod Genet 33(11):1459–1466. https://doi.org/10.1007/s10815-016-0760-y
Tan Y, Yin X, Zhang S, Jiang H, Tan K, Li J et al (2014) Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. GigaScience 3(1):30. https://doi.org/10.1186/2047-217X-3-30
Xu J, Zhang Z, Niu W, Yang Q, Yao G, Shi S et al (2017) Mapping allele with resolved carrier status of Robertsonian and reciprocal translocation in human preimplantation embryos. Proc Natl Acad Sci USA 114(41):E8695–E8702. https://doi.org/10.1073/pnas.1715053114
Munné S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J (2000) Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril 73(6):1209–1218. https://doi.org/10.1016/S0015-0282(00)00495-7
Beyer CE, Willats E (2017) Natural selection between day 3 and day 5/6 PGD embryos in couples with reciprocal or Robertsonian translocations. J Assist Reprod Genet 34(11):1483–1492. https://doi.org/10.1007/s10815-017-1009-0
Young D, Klepacka D, McGarvey M, Schoolcraft WB, Katz-Jaffe MG (2019) Infertility patients with chromosome inversions are not susceptible to an inter-chromosomal effect. J Assist Reprod Genet 36(3):509–616. https://doi.org/10.1007/s10815-018-1376-1
Tur-Kaspa I (2012) Clinical management of in vitro fertilization with preimplantation genetic diagnosis. Semin Reprod Med 30(4):309–322. https://doi.org/10.1055/s-0032-1313910
Li G, Niu W, Jin H, Xu J, Song W, Guo Y et al (2018) Importance of embryo aneuploidy screening in preimplantation genetic diagnosis for monogenic diseases using the karyomap gene chip. Sci Rep 8(1):3139. https://doi.org/10.1038/s41598-018-21094-6
Renwick P, Trussler J, Lashwood A, Braude P, Ogilvie CM (2010) Preimplantation genetic haplotyping: 127 diagnostic cycles demonstrating a robust, efficient alternative to direct mutation testing on single cells. Reprod Biomed Online 20(4):470–476. https://doi.org/10.1016/j.rbmo.2010.01.006
Goldman KN, Nazem T, Berkeley A, Palter S, Grifo JA (2016) Preimplantation genetic diagnosis (PGD) for monogenic disorders: the value of concurrent aneuploidy screening. J Genet Couns 25(6):1327–1337. https://doi.org/10.1007/s10897-016-9975-4
Kane SC, Willats E, Moura SBMEH, Hyett J, da Silva Costa F (2016) Pre-implantation genetic screening techniques: implications for clinical prenatal diagnosis. Fetal Diagn Ther 40(4):241–254. https://doi.org/10.1159/000449381
Wang YZ, Ding CH, Wang J, Zeng YH, Zhou W, Li R et al (2017) Number of blastocysts biopsied as a predictive indicator to obtain at least one normal/balanced embryo following preimplantation genetic diagnosis with single nucleotide polymorphism microarray in translocation cases. J Assist Reprod Genet 34(1):51–59. https://doi.org/10.1007/s10815-016-0831-0
Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM et al (2014) Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril 101(4):967–973. https://doi.org/10.1016/j.fertnstert.2013.12.026
Magnusson A, Kallen K, Thurin-Kjellberg A, Bergh C (2018) The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod 33(1):58–64. https://doi.org/10.1093/humrep/dex334
Zech J, Brandao A, Zech M, Lugger K, Neururer S, Ulmer H et al (2018) Elective frozen-thawed embryo transfer (FET) in women at risk for ovarian hyperstimulation syndrome. Reprod Biol 18(1):46–52. https://doi.org/10.1016/j.repbio.2017.12.004
Gurbuz AS, Gode F, Ozcimen N, Isik AZ (2014) Gonadotrophin-releasing hormone agonist trigger and freeze-all strategy does not prevent severe ovarian hyperstimulation syndrome: a report of three cases. Reprod Biomed Online 29(5):541–544. https://doi.org/10.1016/j.rbmo.2014.07.022
Shapiro BS, Richter KS, Harris DC, Daneshmand ST (2002) Influence of patient age on the growth and transfer of blastocyst-stage embryos. Fertil Steril 77(4):700–705. https://doi.org/10.1016/S0015-0282(01)03251-4
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR et al (2014) The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 101(3):656-663.e1. https://doi.org/10.1016/j.fertnstert.2013.11.004
Acknowledgements
The authors would like to thank all the patients.
Funding
This study was sponsored by the National Natural Science Foundation of China (81771537) and Jiangsu Province Social Development Project (SBE2019740743).
Author information
Authors and Affiliations
Contributions
JZ conceived and designed the study. YC, MD, and TZ collected the data. YC and JZ analyzed the data and drafted the manuscript. FL and ZD performed the PGT experiments. YS assisted with data analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest statement
Authors declare no conflict of interest with respect to the authorship and/or publication of this article.
Ethics approval and consent to participate
The Nanjing Drum Tower Hospital Research Ethics Committee approved this study. The datasets were anonymous; therefore, the requirement for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cai, Y., Ding, M., Zhang, Y. et al. A mathematical model for predicting the number of transferable blastocysts in next-generation sequencing-based preimplantation genetic testing. Arch Gynecol Obstet 305, 241–249 (2022). https://doi.org/10.1007/s00404-021-06050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06050-6