Abstract
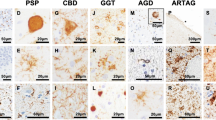
The frontotemporal tauopathies all deposit abnormal tau protein aggregates, but often of only certain isoforms and in distinguishing pathologies of five main types (neuronal Pick bodies, neurofibrillary tangles, astrocytic plaques, tufted astrocytes, globular glial inclusions and argyrophilic grains). In those with isoform specific tau aggregates glial pathologies are substantial, even though there is limited evidence that these cells normally produce tau protein. This review will assess the differentiating features and clinicopathological correlations of the frontotemporal tauopathies, the genetic predisposition for these different pathologies, their neuroanatomical selectivity, current observations on how they spread through the brain, and any potential contributing cellular and molecular changes. The findings show that diverse clinical phenotypes relate most to the brain region degenerating rather than the type of pathology involved, that different regions on the MAPT gene and novel risk genes are associated with specific tau pathologies, that the 4-repeat glial tauopathies do not follow individual patterns of spreading as identified for neuronal pathologies, and that genetic and pathological data indicate that neuroinflammatory mechanisms are involved. Each pathological frontotemporal tauopathy subtype with their distinct pathological features differ substantially in the cell type affected, morphology, biochemical and anatomical distribution of inclusions, a fundamental concept central to future success in understanding the disease mechanisms required for developing therapeutic interventions. Tau directed therapies targeting genetic mechanisms, tau aggregation and pathological spread are being trialled, although biomarkers that differentiate these diseases are required. Suggested areas of future research to address the regional and cellular vulnerabilities in frontotemporal tauopathies are discussed.




Similar content being viewed by others
References
Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B et al (2013) Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol 126:537–544. https://doi.org/10.1007/s00401-013-1171-0
Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H et al (2014) A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127:667–683
Ahmed Z, Doherty KM, Silveira-Moriyama L, Bandopadhyay R, Lashley T, Mamais A, Hondhamuni G, Wray S, Newcombe J, O’Sullivan SS et al (2011) Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122:415–428. https://doi.org/10.1007/s00401-011-0857-4
Alcolea D, Vilaplana E, Suarez-Calvet M, Illan-Gala I, Blesa R, Clarimon J, Llado A, Sanchez-Valle R et al (2017) CSF sAPPbeta, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 89:178–188. https://doi.org/10.1212/WNL.0000000000004088
Ali F, Josephs KA (2018) Corticobasal degeneration: key emerging issues. J Neurol 265:439–445. https://doi.org/10.1007/s00415-017-8644-3
Ali F, Martin PR, Botha H, Ahlskog JE, Bower JH, Masumoto JY, Maraganore D, Hassan A et al (2019) Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord. https://doi.org/10.1002/mds.27619
Allen M, Burgess JD, Ballard T, Serie D, Wang X, Younkin CS, Sun Z, Kouri N, Baheti S et al (2016) Gene expression, methylation and neuropathology correlations at progressive supranuclear palsy risk loci. Acta Neuropathol 132:197–211
Allen M, Wang X, Serie DJ, Strickland SL, Burgess JD, Koga S, Younkin CS, Nguyen TT, Malphrus KG et al (2018) Divergent brain gene expression patterns associate with distinct cell-specific tau neuropathology traits in progressive supranuclear palsy. Acta Neuropathol 136:709–727
Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C et al (2001) Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 101:167–173
Arima K (2006) Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 26:475–483
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M et al (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503. https://doi.org/10.1212/WNL.0b013e31827f0fd1
Arnold SE, Han LY, Clark CM, Grossman M, Trojanowski JQ (2000) Quantitative neurohistological features of frontotemporal degeneration. Neurobiol Aging 21:913–919
Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang LS, Baek Y, Wolk DA, Lee EB, Miller BL et al (2013) Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol 521:4339–4355. https://doi.org/10.1002/cne.23430
Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8:663–672. https://doi.org/10.1038/nrn2194
Beretti F, Ardizzoni A, Cermelli C, Guida M, Maraldi T, Pietrosemoli P, Paulone S, De Pol A, Blasi E et al (2017) Apoptosis and inflammatory response in human astrocytes are induced by a transmissible cytotoxic agent of neurological origin. New Microbiol 40:27–32
Bonham LW, Karch CM, Fan CC, Tan C, Geier EG, Wang Y (2018) CXCR16 involvement in neurodegenerative diseases. Transl Psychiatry 8:73
Borrego-Ecija S, Morgado J, Palencia-Madrid L, Grau-Rivera O, Rene R, Hernandez I, Almenar C, Balasa M, Antonell A et al (2017) Frontotemporal dementia caused by the p301l mutation in the mapt gene: clinicopathological features of 13 cases from the same geographical origin in Barcelona, Spain. Dement Geriatr Cogn Disord 44:213–221. https://doi.org/10.1159/000480077
Botez G, Probst A, Ipsen S, Tolnay M (1999) Astrocytes expressing hyperphosphorylated tau protein without glial fibrillary tangles in argyrophilic grain disease. Acta Neuropathol 98:251–256
Braak H, Braak E (1998) Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm 105:801–819
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. https://doi.org/10.1097/NEN.0b013e318232a379
Broce I, Karch CM, Wen N, Fan CC, Wang Y, Tan CH, Kouri N, Ross OA et al (2018) Immune-related genetic enrichment in frontotemporal dementia: an analysis of genome-wide association studies. PLoS Med 15:e1002487. https://doi.org/10.1371/journal.pmed.1002487
Broe M, Kril J, Halliday GM (2004) Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain 127:2214–2220. https://doi.org/10.1093/brain/awh250
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33:95–130
Buee L, Delacourte A (1999) Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 9:681–693
Buee Scherrer V, Hof PR, Buee L, Leveugle B, Vermersch P, Perl DP, Olanow CW et al (1996) Hyperphosphorylated tau proteins differentiate corticobasal degeneration and Pick’s disease. Acta Neuropathol 91:351–359
Burrell JR, Forrest S, Bak TH, Hodges JR, Halliday GM, Kril JJ (2016) Expanding the phenotypic associations of globular glial tau subtypes. Alzheimers Dement (Amst) 4:6–13. https://doi.org/10.1016/j.dadm.2016.03.006
Burrell JR, Hodges JR, Rowe JB (2014) Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord 29:684–693. https://doi.org/10.1002/mds.25872
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562:578–582. https://doi.org/10.1038/s41586-018-0543-y
Caffrey TM, Joachim C, Wade-Martins R (2008) Haplotype-specific expression of the N-terminal exons 2 and 3 at the human MAPT locus. Neurobiol Aging 29:1923–1929. https://doi.org/10.1016/j.neurobiolaging.2007.05.002
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. https://doi.org/10.1007/s00401-007-0237-2
Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, Halliday GM (2014) New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry 85:865–870. https://doi.org/10.1136/jnnp-2013-306948
Combs B, Gamblin TC (2012) FTDP-17 tau mutations induce distinct effects on aggregation and microtubule interactions. Biochemistry 51:8597–8607. https://doi.org/10.1021/bi3010818
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, Buee L, Maurage CA, Pasquier F (2010) Prediction of pathology in primary progressive language and speech disorders. Neurology 74:42–49. https://doi.org/10.1212/WNL.0b013e3181c7198e
DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T et al (2017) Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aag0481
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400. https://doi.org/10.1097/WCO.0b013e32833be924
Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45:384–389. https://doi.org/10.1007/s12031-011-9589-0
Durkee CA, Araque A (2019) Diversity and Specificity of astrocyte-neuron communication. Neuroscience 396:73–78. https://doi.org/10.1016/j.neuroscience.2018.11.010
Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M, Halliday G, Neumann M, Spillantini MG et al (2015) PART is part of Alzheimer disease. Acta Neuropathol 129:749–756. https://doi.org/10.1007/s00401-015-1390-7
Evers MM, Toonen LJ, van Roon-Mom WM (2015) Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev 87:90–103. https://doi.org/10.1016/j.addr.2015.03.008
Fagan AM, Perrin RJ (2012) Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med 6:455–476. https://doi.org/10.2217/bmm.12.42
Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, Crowther RA, Ghetti B et al (2018) Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561:137–140. https://doi.org/10.1038/s41586-018-0454-y
Ferrari R, Ryten M, Simone R, Trabzuni D, Nicolaou N, Hondhamuni G et al (2014) Assessment of common variability and expression quantitative trait loci for genome-wide associations for progressive supranuclear palsy. Neurobiol Aging 35:e1511–e1512. https://doi.org/10.1016/j.neurobiolaging.2014.01.010
Ferrer I, Lopez-Gonzalez I, Carmona M, Arregui L, Dalfo E, Torrejon-Escribano B, Diehl R et al (2014) Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol 73:81–97. https://doi.org/10.1097/nen.0000000000000030
Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432. https://doi.org/10.1093/brain/awm305
Forrest SL, Halliday GM, McCann H, McGeachie AB, McGinley CV, Hodges JR et al (2019) Heritability in frontotemporal tauopathies. Alzheimers Dement (Amst) 11:115–124. https://doi.org/10.1016/j.dadm.2018.12.001
Forrest SL, Kril JJ, Stevens CH, Kwok JB, Hallupp M, Kim WS, Huang Y, McGinley CV et al (2018) Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 141:521–534
Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR et al (2007) FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 130:2616–2635. https://doi.org/10.1093/brain/awm177
Franzmeier N, Rubinski A, Neitzel J, Kim Y, Damm A, Na DL et al (2019) Functional connectivity associated with tau levels in ageing, Alzheimer’s, and small vessel disease. Brain 142:1093–1107
Fu H, Hardy J, Duff KE (2018) Selective vulnerability in neurodegenerative diseases. Nat Neurosci 21:1350–1358. https://doi.org/10.1038/s41593-018-0221-2
Fujioka S, Sanchez Contreras MY, Strongosky AJ, Ogaki K, Whaley NR, Tacik PM et al (2015) Three sib-pairs of autopsy-confirmed progressive supranuclear palsy. Parkinsonism Relat Disord 21:101–105. https://doi.org/10.1016/j.parkreldis.2014.10.028
Furman JL, Vaquer-Alicea J, White CL 3rd, Cairns NJ, Nelson PT, Diamond MI (2017) Widespread tau seeding activity at early Braak stages. Acta Neuropathol 133:91–100. https://doi.org/10.1007/s00401-016-1644-z
Gasca-Salas C, Masellis M, Khoo E, Shah BB, Fisman D, Lang AE, Kleiner-Fisman G (2016) Characterization of movement disorder phenomenology in genetically proven, familial frontotemporal lobar degeneration: a systematic review and meta-analysis. PLoS One 11:e0153852. https://doi.org/10.1371/journal.pone.0153852
Gauthier-Kemper A, Weissmann C, Golovyashkina N, Sebo-Lemke Z, Drewes G, Gerke V, Heinisch JJ et al (2011) The frontotemporal dementia mutation R406 W blocks tau’s interaction with the membrane in an annexin A2-dependent manner. J Cell Biol 192:647–661. https://doi.org/10.1083/jcb.201007161
Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M (2015) Invited review: frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol 41:24–46. https://doi.org/10.1111/nan.12213
Gil MJ, Manzano MS, Cuadrado ML, Fernandez C, Gomez E, Matesanz C et al (2018) Argyrophilic grain pathology in frontotemporal lobar degeneration: demographic, clinical, neuropathological, and genetic features. J Alzheimers Dis 63:1109–1117. https://doi.org/10.3233/JAD-171115
Gil MJ, Manzano MS, Cuadrado ML, Fernandez C, Gomez E, Matesanz C, Calero M et al (2018) Frontotemporal lobar degeneration: study of a clinicopathological cohort. J Clin Neurosci 58:172–180. https://doi.org/10.1016/j.jocn.2018.10.024
Gil MJ, Serrano S, Manzano MS, Cuadrado ML, Gomez E, Rabano A (2019) Argyrophilic grain disease presenting as behavioral frontotemporal dementia. Clin Neuropathol 38:8–13. https://doi.org/10.5414/np301122
Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, Lomen-Hoerth C, Wilhelmsen KC, Lee VM et al (2005) Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65:1817–1819. https://doi.org/10.1212/01.wnl.0000187068.92184.63
Gunawardana CG, Mehrabian M, Wang X, Mueller I, Lubambo IB, Jonkman JE, Wang H et al (2015) The human Tau interactome: binding to the ribonucleoproteome, and impaired binding of the proline-to-leucine mutant at position 301 (P301L) to chaperones and the proteasome. Mol Cell Proteom 14:3000–3014. https://doi.org/10.1074/mcp.M115.050724
Heckman MG, Brennan RR, Labbe C, Soto AI, Koga S, DeTure MA, Murray ME et al (2019) Association of MAPT subhaplotypes with risk of progressive supranuclear palsy and severity of tau pathology. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2019.0250
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P et al (2010) Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain 133:300–306. https://doi.org/10.1093/brain/awp248
Hoglinger GU (2018) Is it useful to classify progressive supranuclear palsy and corticobasal degeneration as different disorders? No. Mov Disord Clin Pract 5:141–144. https://doi.org/10.1002/mdc3.12582
Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L et al (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 43:699–705. https://doi.org/10.1038/ng.859
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864
Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI et al (1998) Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282:1914–1917
Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE et al (2016) Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 79:272–287. https://doi.org/10.1002/ana.24559
Ittner A, Ittner LM (2018) Dendritic Tau in Alzheimer’s disease. Neuron 99:13–27. https://doi.org/10.1016/j.neuron.2018.06.003
Jadhav S, Avila J, Scholl M, Kovacs GG, Kovari E, Skrabana R et al (2019) A walk through tau therapeutic strategies. Acta Neuropathol Commun 7:22. https://doi.org/10.1186/s40478-019-0664-z
Jellinger KA (2018) Different patterns of hippocampal tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 136:811–813. https://doi.org/10.1007/s00401-018-1894-z
Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF et al (2015) PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 129:757–762. https://doi.org/10.1007/s00401-015-1407-2
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117. https://doi.org/10.1007/s00401-006-0156-7
Jiskoot LC, Bocchetta M, Nicholas JM, Cash DM, Thomas D, Modat M et al (2018) Presymptomatic white matter integrity loss in familial frontotemporal dementia in the GENFI cohort: a cross-sectional diffusion tensor imaging study. Ann Clin Transl Neurol 5:1025–1036
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM et al (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122:137–153. https://doi.org/10.1007/s00401-011-0839-6
Josephs KA, Katsuse O, Beccano-Kelly DA, Lin WL, Uitti RJ, Fujino Y et al (2006) Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol 65:396–405. https://doi.org/10.1097/01.jnen.0000218446.38158.61
Josephs KA, Whitwell JL, Parisi JE, Knopman DS, Boeve BF, Geda YE et al (2008) Argyrophilic grains: a distinct disease or an additive pathology? Neurobiol Aging 29:566–573
Kaufman SK, Del Tredici K, Thomas TL, Braak H, Diamond MI (2018) Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 136:57–67
Kersaitis C, Halliday GM, Kril JJ (2004) Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without pick bodies. Acta Neuropathol 108:515–523. https://doi.org/10.1007/s00401-004-0917-0
Kielb S, Cook A, Wieneke C, Rademaker A, Bigio EH, Mesulam MM et al (2016) Neuropathologic associations of learning and memory in primary progressive aphasia. JAMA Neurol 73:846–852. https://doi.org/10.1001/jamaneurol.2016.0880
Kouri N, Oshima K, Takahashi M, Murray ME, Ahmed Z, Parisi JE et al (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125:741–752. https://doi.org/10.1007/s00401-013-1087-8
Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A et al (2015) Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun 6:7247. https://doi.org/10.1038/ncomms8247
Kovacs GG (2018) Tauopthies. In: Kovacs GG, Alafuzoff I (eds) Handbook of clinical neurology, 3rd edn. Neuropathology, vol 145. Elsevier, Amsterdam, pp 355–368
Kovacs GG, Budka H (2010) Current concepts of neuropathological diagnostics in practice: neurodegenerative diseases. Clin Neuropathol 29:271–288
Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, Cairns NJ et al (2016) Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 131:87–102. https://doi.org/10.1007/s00401-015-1509-x
Kovacs GG, Lee VM, Trojanowski JQ (2017) Protein astrogliopathies in human neurodegenerative diseases and aging. Brain Pathol 27:675–690. https://doi.org/10.1111/bpa.12536
Kovacs GG, Pittman A, Revesz T, Luk C, Lees A, Kiss E et al (2008) MAPT S305I mutation: implications for argyrophilic grain disease. Acta Neuropathol 116:103–118. https://doi.org/10.1007/s00401-007-0322-6
Kovacs GG, Robinson JL, Xie SX, Lee EB, Grossman M, Wolk DA et al (2017) Evaluating the patterns of aging-related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. J Neuropathol Exp Neurol 76:270–288. https://doi.org/10.1093/jnen/nlx007
Kovacs GG, Rozemuller AJ, van Swieten JC, Gelpi E, Majtenyi K, Al-Sarraj S et al (2013) Neuropathology of the hippocampus in FTLD-Tau with Pick bodies: a study of the BrainNet Europe Consortium. Neuropathol Appl Neurobiol 39:166–178. https://doi.org/10.1111/j.1365-2990.2012.01272.x
Kovacs GG, Xie SX, Robinson JL, Lee EB, Smith DH, Schuck T et al (2018) Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Commun 6:50
Lang AE (2003) Corticobasal degeneration: selected developments. Mov Disord 18(Suppl 6):S51–56. https://doi.org/10.1002/mds.10563
Lashley T, Rohrer JD, Mead S, Revesz T (2015) Review: an update on clinical, genetic and pathological aspects of frontotemporal lobar degenerations. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12250
Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK et al (2017) Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol. https://doi.org/10.1007/s00401-017-1679-9
Ling H, de Silva R, Massey LA, Courtney R, Hondhamuni G, Bajaj N et al (2014) Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathol Appl Neurobiol 40:149–163. https://doi.org/10.1111/nan.12037
Ling H, Kovacs GG, Vonsattel JP, Davey K, Mok KY, Hardy J et al (2016) Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 139:3237–3252. https://doi.org/10.1093/brain/aww256
Ling H, Macerollo A (2018) Is it useful to classify PSP and CBD as different disorders? Yes. Mov Disord Clin Pract 5:145–148. https://doi.org/10.1002/mdc3.12581
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS et al (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105
Martinez-Maldonado A, Luna-Munoz J, Ferrer I (2016) Incidental corticobasal degeneration. Neuropathol Appl Neurobiol 42:659–663. https://doi.org/10.1111/nan.12339
Mathew R, Bak TH, Hodges JR (2012) Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry 83:405–410. https://doi.org/10.1136/jnnp-2011-300875
McMillan CT, Toledo JB, Avants BB, Cook PA, Wood EM, Suh E et al (2014) Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiol Aging 35:1473–1482. https://doi.org/10.1016/j.neurobiolaging.2013.11.029
Meeter LH, Kaat LD, Rohrer JD, van Swieten JC (2017) Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol 13:406–419. https://doi.org/10.1038/nrneurol.2017.75
Melki R (2018) How the shapes of seeds can influence pathology. Neurobiol Dis 109:201–208. https://doi.org/10.1016/j.nbd.2017.03.011
Milenkovic I, Kovacs GG (2013) Incidental corticobasal degeneration in a 76-year-old woman. Clin Neuropathol 32:69–72. https://doi.org/10.5414/NP300515
Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R (2018) Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: a review. Front Neurol 9:692. https://doi.org/10.3389/fneur.2018.00692
Morris HR, Baker M, Yasojima K, Houlden H, Khan MN, Wood NW et al (2002) Analysis of tau haplotypes in Pick’s disease. Neurology 59:443–445
Murray ME, Kouri N, Lin WL, Jack CR Jr, Dickson DW, Vemuri P et al (2014) Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res Ther 6:1. https://doi.org/10.1186/alzrt231
Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q et al (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17:e12840. https://doi.org/10.1111/acel.12840
Niethammer M, Tang CC, Feigin A, Allen PJ, Heinen L, Hellwig S et al (2014) A disease-specific metabolic brain network associated with corticobasal degeneration. Brain 137:3036–3046. https://doi.org/10.1093/brain/awu256
Nogami A, Yamazaki M, Saito Y, Hatsuta H, Sakiyama Y, Takao M et al (2015) Early stage of progressive supranuclear palsy: a neuropathological study of 324 consecutive autopsy cases. J Nippon Med Sch 82:266–273. https://doi.org/10.1272/jnms.82.266
Osaki Y, Ben-Shlomo Y, Lees AJ, Daniel SE, Colosimo C, Wenning G, Quinn N (2004) Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord 19:181–189. https://doi.org/10.1002/mds.10680
Pasqualetti G, Brooks DJ, Edison P (2015) The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep 15:17. https://doi.org/10.1007/s11910-015-0531-7
Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A et al (2017) Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 140:3329–3345. https://doi.org/10.1093/brain/awx254
Piacentini R, Li Puma DD, Mainardi M, Lazzarino G, Tavazzi B, Arancio O et al (2017) Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia 65:1302–1316. https://doi.org/10.1002/glia.23163
Piguet O, Halliday GM, Reid WG, Casey B, Carman R, Huang Y et al (2011) Clinical phenotypes in autopsy-confirmed Pick disease. Neurology 76:253–259. https://doi.org/10.1212/WNL.0b013e318207b1ce
Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L et al (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825. https://doi.org/10.1002/ana.410430617
Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, San Martin-Paniello C, Clarimon J, Belbin O et al (2017) YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J Neuroinflammation 14:118. https://doi.org/10.1186/s12974-017-0893-7
Rademakers R, Neumann M, Mackenzie IR (2013) Advances in understanding the molecular basis of frontotemporal dementia (vol 8, p 423, 2012). Nat Rev Neurol 9:423–434. https://doi.org/10.1038/Nrneurol.2013.76
Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW et al (2016) Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol 73:1078–1088
Reed LA, Wszolek ZK, Hutton M (2001) Phenotypic correlations in FTDP-17. Neurobiol Aging 22:89–107
Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ et al (2014) The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 29:1758–1766. https://doi.org/10.1002/mds.26054
Rodriguez RD, Suemoto CK, Molina M, Nascimento CF, Leite RE, de Lucena Ferretti-Rebustini RE et al (2016) Argyrophilic grain disease: demographics, clinical, and neuropathological features from a large autopsy study. J Neuropathol Exp Neurol 75:628–635. https://doi.org/10.1093/jnen/nlw034
Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM et al (2011) Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134:2565–2581
Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L et al (2015) Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the genetic frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 14:253–262. https://doi.org/10.1016/S1474-4422(14)70324-2
Ronnback A, Nennesmo I, Tuominen H, Grueninger F, Viitanen M, Graff C et al (2014) Neuropathological characterization of two siblings carrying the MAPT S305S mutation demonstrates features resembling argyrophilic grain disease. Acta Neuropathol 127:297–298. https://doi.org/10.1007/s00401-013-1229-z
Saito Y, Nakahara K, Yamanouchi H, Murayama S (2002) Severe involvement of ambient gyrus in dementia with grains. J Neuropathol Exp Neurol 61:789–796
Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y et al (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63:911–918
Sakers K, Lake AM, Khazanchi R, Ouwenga R, Vasek MJ, Dani A et al (2017) Astrocytes locally translate transcripts in their peripheral processes. Proc Natl Acad Sci USA 114:E3830–E3838. https://doi.org/10.1073/pnas.1617782114
Santa-Maria I, Haggiagi A, Liu X, Wasserscheid J, Nelson PT, Dewar K et al (2012) The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol 124:693–704. https://doi.org/10.1007/s00401-012-1017-1
Santello M, Toni N, Volterra A (2019) Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22:154–166. https://doi.org/10.1038/s41593-018-0325-8
Scaravilli T, Tolosa E, Ferrer I (2005) Progressive supranuclear palsy and corticobasal degeneration: lumping versus splitting. Mov Disord 20(Suppl 12):S21–28. https://doi.org/10.1002/mds.20536
Shoeibi A, Olfati N, Litvan I (2018) Preclinical, phase I, and phase II investigational clinical trials for treatment of progressive supranuclear palsy. Expert Opin Investig Drugs 27:349–361. https://doi.org/10.1080/13543784.2018.1460356
Sica RE (2015) Could astrocytes be the primary target of an offending agent causing the primary degenerative diseases of the human central nervous system? A hypothesis. Med Hypotheses 84:481–489. https://doi.org/10.1016/j.mehy.2015.02.004
Skaper SD, Facci L, Zusso M, Giusti P (2018) An Inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci 12:72. https://doi.org/10.3389/fncel.2018.00072
Smith R, Puschmann A, Scholl M, Ohlsson T, van Swieten J, Honer M et al (2016) 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain 139:2372–2379
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12:609–622. https://doi.org/10.1016/s1474-4422(13)70090-5
Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F et al (2017) Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 81:430–443
Staffaroni AM, Ljubenkov PA, Kornak J, Cobigo Y, Datta S, Marx G et al (2019) Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain 142:443–459. https://doi.org/10.1093/brain/awy319
Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J et al (2005) A common inversion under selection in Europeans. Nat Genet 37:129–137. https://doi.org/10.1038/ng1508
Strafela P, Plesko J, Magdic J, Koritnik B, Zupan A, Glavac D et al (2018) Familial tauopathy with P364S MAPT mutation: clinical course, neuropathology and ultrastructure of neuronal tau inclusions. Neuropathol Appl Neurobiol 44:550–562. https://doi.org/10.1111/nan.12456
Sud R, Geller ET, Schellenberg GD (2014) Antisense-mediated Exon skipping decreases Tau Protein Expression: a potential therapy for tauopathies. Mol Ther Nucleic Acids 3:e180
Tacik P, DeTure M, Lin WL, Sanchez Contreras M, Wojtas A, Hinkle KM et al (2015) A novel tau mutation, p. K317N, causes globular glial tauopathy. Acta Neuropathol 130:199–214. https://doi.org/10.1007/s00401-015-1425-0
Tacik P, Sanchez-Contreras M, DeTure M, Murray ME, Rademakers R, Ross OA et al (2017) Clinicopathologic heterogeneity in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) due to microtubule-associated protein tau (MAPT) p. P301L mutation, including a patient with globular glial tauopathy. Neuropathol Appl Neurobiol 43:200–214
Teunissen CE, Elias N, Koel-Simmelink MJ, Durieux-Lu S, Malekzadeh A, Pham TV et al (2016) Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement (Amst) 2:86–94. https://doi.org/10.1016/j.dadm.2015.12.004
Thal DR, von Arnim CA, Griffin WS, Mrak RE, Walker L, Attems J et al (2015) Frontotemporal lobar degeneration FTLD-tau: preclinical lesions, vascular, and Alzheimer-related co-pathologies. J Neural Transm (Vienna) 122:1007–1018. https://doi.org/10.1007/s00702-014-1360-6
Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R et al (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Tolnay M, Spillantini MG, Goedert M, Ulrich J, Langui D, Probst A et al (1997) Argyrophilic grain disease: widespread hyperphosphorylation of tau protein in limbic neurons. Acta Neuropathol 93:477–484
Trabzuni D, Wray S, Vandrovcova J, Ramasamy A, Walker R, Smith C et al (2012) MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet 21:4094–4103. https://doi.org/10.1093/hmg/dds238
Uchihara T (2007) Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol 113:483–499
Uchihara T, Mizusawa H, Tsuchiya K, Kondo H, Oda T, Ikeda K et al (1998) Discrepancy between tau immunoreactivity and argyrophilia by the Bodian method in neocortical neurons of corticobasal degeneration. Acta Neuropathol 96:553–557
Uchihara T, Nakamura A, Shibuya K, Yagishita S (2011) Specific detection of pathological three-repeat tau after pretreatment with potassium permanganate and oxalic acid in PSP/CBD brains. Brain Pathol 21:180–188. https://doi.org/10.1111/j.1750-3639.2010.00433.x
Uchihara T, Nakamura A, Yamazaki M, Mori O (2000) Tau-positive neurons in corticobasal degeneration and Alzheimer’s disease–distinction by thiazin red and silver impregnations. Acta Neuropathol 100:385–389
van Swieten JC, Stevens M, Rosso SM, Rizzu P, Joosse M, de Koning I et al (1999) Phenotypic variation in hereditary frontotemporal dementia with tau mutations. Ann Neurol 46:617–626
Vandenberghe R (2016) Classification of the primary progressive aphasias: principles and review of progress since 2011. Alzheimers Res Ther 8:16. https://doi.org/10.1186/s13195-016-0185-y
Walsh DM, Selkoe DJ (2016) A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci 17:251–260. https://doi.org/10.1038/nrn.2016.13
Weller RO, Hawkes CA, Carare RO, Hardy J (2015) Does the difference between PART and Alzheimer’s disease lie in the age-related changes in cerebral arteries that trigger the accumulation of Abeta and propagation of tau? Acta Neuropathol 129:763–766. https://doi.org/10.1007/s00401-015-1416-1
Whitwell JL, Hoglinger GU, Antonini A, Bordelon Y, Boxer AL, Colosimo C et al (2017) Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord 32:955–971. https://doi.org/10.1002/mds.27038
Whitwell JL, Josephs KA (2012) Neuroimaging in frontotemporal lobar degeneration–predicting molecular pathology. Nat Rev Neurol 8:131–142. https://doi.org/10.1038/nrneurol.2012.7
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ et al (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130:1566–1576. https://doi.org/10.1093/brain/awm104
Wood EM, Falcone D, Suh E, Irwin DJ, Chen-Plotkin AS, Lee EB et al (2013) Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol 70:1411–1417. https://doi.org/10.1001/jamaneurol.2013.3956
Wood R, Moodley K, Hodges JR, Allinson K, Spillantini MG, Chan D et al (2016) Slowly progressive behavioural presentation in two UK cases with the R406 W MAPT mutation. Neuropathol Appl Neurobiol 42:291–295. https://doi.org/10.1111/nan.12247
Yang Q, Wang EY, Huang XJ, Qu WS, Zhang L, Xu JZ et al (2011) Blocking epidermal growth factor receptor attenuates reactive astrogliosis through inhibiting cell cycle progression and protects against ischemic brain injury in rats. J Neurochem 119:644–653. https://doi.org/10.1111/j.1471-4159.2011.07446.x
Yokota O, Tsuchiya K, Arai T, Yagishita S, Matsubara O, Mochizuki A, Tamaoka A et al (2009) Clinicopathological characterization of Pick’s disease versus frontotemporal lobar degeneration with ubiquitin/TDP-43-positive inclusions. Acta Neuropathol 117:429–444. https://doi.org/10.1007/s00401-009-0493-4
Yokoyama JS, Karch CM, Fan CC, Bonham LW, Kouri N, Ross OA et al (2017) Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol 133:825–837. https://doi.org/10.1007/s00401-017-1693-y
Yoshida H, Crowther RA, Goedert M (2002) Functional effects of tau gene mutations deltaN296 and N296H. J Neurochem 80:548–551
Yoshida K, Hata Y, Kinoshita K, Takashima S, Tanaka K, Nishida N et al (2017) Incipient progressive supranuclear palsy is more common than expected and may comprise clinicopathological subtypes: a forensic autopsy series. Acta Neuropathol 133:809–823. https://doi.org/10.1007/s00401-016-1665-7
Yoshida M (2006) Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology 26:457–470
Young AL, Marinescu RV, Oxtoby NP, Bocchetta M, Yong K, Firth NC, Cash DM et al (2018) Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with subtype and stage inference. Nat Commun 9:4273. https://doi.org/10.1038/s41467-018-05892-0
Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA et al (2014) FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol 261:710–716. https://doi.org/10.1007/s00415-014-7256-4
Zetterberg H, van Swieten JC, Boxer AL, Rohrer JD (2019) Review: fluid biomarkers for frontotemporal dementias. Neuropathol Appl Neurobiol 45:81–87. https://doi.org/10.1111/nan.12530
Zhang CC, Zhu JX, Wan Y, Tan L, Wang HF, Yu JT et al (2017) Meta-analysis of the association between variants in MAPT and neurodegenerative diseases. Oncotarget 8:44994–45007. https://doi.org/10.18632/oncotarget.16690
Acknowledgements
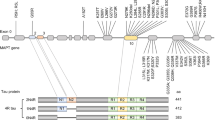
The authors wish to thank Ms Heidi Cartwright for assistance with preparation of figures and the staff of the Sydney Brain Bank (supported by the University of New South Wales and Neuroscience Research Australia) and the NSW Brain Tissue Resource Centre (supported by the National Institute on Alcohol Abuse and Alcoholism, NIHR28AA012725) for initial characterisation of the cases used to prepare the sections for the figurework. We also thank Dr Janet Van Eersel and Professor Lars Ittner from the Dementia Research Centre, Macquarie University, for providing the tau western blot used in Fig. 4.
Funding
This work was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neurone disease, from NHMRC of Australia program grants (#1132524). GH is a NHMRC Senior Principal Research Fellow (#1079679).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Forrest, S.L., Kril, J.J. & Halliday, G.M. Cellular and regional vulnerability in frontotemporal tauopathies. Acta Neuropathol 138, 705–727 (2019). https://doi.org/10.1007/s00401-019-02035-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-019-02035-7