Abstract
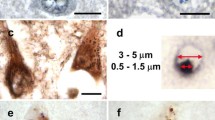
To elucidate the maturation process of TDP-43-positive neuronal inclusions, we immunohistochemically and immunoelectron-microscopically examined multiple areas from the brain and spinal cord from ten patients with amyotrophic lateral sclerosis (ALS) and 25 control subjects. TDP-43 immunohistochemistry demonstrated three types of inclusions in ALS: skein-like, round, and dot-like inclusions. Skein-like inclusions were found in all cases of ALS. Dot-like inclusions were found in the anterior horn in seven cases of ALS, all of whom had round inclusions, but not in cases without round inclusions. In addition, careful examination revealed two types of diffuse punctate cytoplasmic staining: linear wisps and punctate granules. Linear wisps were present in all cases of ALS but in none of 25 controls. In contrast, punctate granules were detected in all cases of ALS as well as in five of 13 normal and in seven of 12 diseased controls. Immunoelectron-microscopy revealed that skein-like inclusions consisted of granule-associated parallel filaments. Round and dot-like inclusions were composed of granulo-filamentous structures. However, punctate granules corresponded to the mitochondria and were not immunostained with anti-ubiquitin, indicating that punctate granules represent cross-reaction. We assumed that linear wisps (“fine skein”) aggregate as thicker and longer threads (“coarse skein”), whereas round inclusions arise from dot-like inclusions. These findings suggest that there are differences in the formation process between skein-like and round inclusions, despite the antigenic and ultrastructural similarities.





Similar content being viewed by others
References
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445. doi:10.1002/ana.21154
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611. doi:10.1016/j.bbrc.2006.10.093
Brandmeir NJ, Geser F, Kwong LK, Zimmerman E, Qian J, Lee VM et al (2008) Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol 115:123–131. doi:10.1007/s00401-007-0315-5
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol 114:5–22. doi:10.1007/s00401-007-0237-2
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240. doi:10.2353/ajpath.2007.070182
Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D et al (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533. doi:10.1007/s00401-006-0189-y
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114:71–79. doi:10.1007/s00401-007-0234-5
Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP (2008) TAR-DNA binding protein 43 in Pick disease. J Neuropathol Exp Neurol 67:62–67. doi:10.1097/nen.0b013e3181609361
Fujita Y, Mizuno Y, Takatama M, Okamoto K (2008) Anterior horn cells with abnormal TDP-43 immunoreactivities show fragmentation of the Golgi apparatus in ALS. J Neurol Sci 269:30–34. doi:10.1016/j.jns.2007.12.016
Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM et al (2008) Pathological TDP-43 in parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 115:133–145. doi:10.1007/s00401-007-0257-y
Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D et al (2008) TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol 63:535–538. doi:10.1002/ana.21344
Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T et al (2007) TDP-43 is deposited in the Guam parkinsonism–dementia complex brains. Brain 130:1386–1394. doi:10.1093/brain/awm065
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K et al (2007) Concurrence of TDP-43, tau and α-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294. doi:10.1016/j.brainres.2007.09.048
Kato S, Takikawa M, Nakashima K, Hirano A, Cleveland DW, Kusaka H et al (2000) New consensus research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 (SOD1) gene mutations: inclusions containing SOD1 in neurons and astrocytes. Amyotroph Lateral Scler Other Motor Neuron Disord 1:163–184. doi:10.1080/14660820050515160
Leigh PN, Anderton BH, Dodson A, Gallo JM, Swash M, Power DM (1988) Ubiquitin deposits in anterior horn cells in motor neurone disease. Neurosci Lett 93:197–203. doi:10.1016/0304-3940(88)90081-X
Leigh PN, Whitwell H, Garofalo O, Buller J, Swash M, Martin JE et al (1991) Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain 114:775–788. doi:10.1093/brain/114.2.775
Lowe J, Lennox G, Jefferson D, Morrell K, McQuire D, Gray T et al (1988) A filamentous inclusion body within anterior horn neurones in motor neurone disease defined by immunocytochemical localisation of ubiquitin. Neurosci Lett 94:203–210. doi:10.1016/0304-3940(88)90296-0
Lowe J, Aldridge F, Lennox G, Doherty F, Jefferson D, Landon M et al (1989) Inclusion bodies in motor cortex and brainstem of patients with motor neurone disease are detected by immunocytochemical localisation of ubiquitin. Neurosci Lett 105:7–13. doi:10.1016/0304-3940(89)90003-7
Lowe J (1994) New pathological findings in amyotrophic lateral sclerosis. J Neurol Sci 124(Suppl):38–51. doi:10.1016/0022-510X(94)90175-9
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH et al (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549. doi:10.1007/s00401-006-0138-9
Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ et al (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434. doi:10.1002/ana.21147
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809. doi:10.1001/archneur.58.11.1803
Murayama S, Ookawa Y, Mori H, Nakano I, Ihara Y, Kuzuhara S et al (1989) Immunocytochemical and ultrastructural study of Lewy body-like hyaline inclusions in familial amyotrophic lateral sclerosis. Acta Neuropathol 78:143–152. doi:10.1007/BF00688202
Murayama S, Mori H, Ihara Y, Bouldin TW, Suzuki K, Tomonaga M (1990) Immunocytochemical and ultrastructural studies of lower motor neurons in amyotrophic lateral sclerosis. Ann Neurol 27:137–148. doi:10.1002/ana.410270208
Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229. doi:10.1007/s00401-007-0261-2
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. doi:10.1126/science.1134108
Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T et al (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Probst A, Taylor KI, Tolnay M (2007) Hippocampal sclerosis dementia: a reappraisal. Acta Neuropathol 114:335–345. doi:10.1007/s00401-007-0262-1
Robertson J, Sanelli T, Xiao S, Yang W, Horne P, Hammond R et al (2007) Lack of TDP-43 abnormalities in mutant SOD1 transgenic mice shows disparity with ALS. Neurosci Lett 420:128–132. doi:10.1016/j.neulet.2007.03.066
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A et al (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352. doi:10.2353/ajpath.2006.060438
Sasaki S, Yamane K, Sakuma H, Maruyama S (1989) Sporadic motor neuron disease with Lewy body-like hyaline inclusions. Acta Neuropathol 78:555–560. doi:10.1007/BF00687719
Sasaki S, Maruyama S (1991) Immunocytochemical and ultrastructural studies of hyaline inclusions in sporadic motor neuron disease. Acta Neuropathol 82:295–301. doi:10.1007/BF00308815
Seelaar H, Schelhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D et al (2007) TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain 130:1375–1385. doi:10.1093/brain/awm024
Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WY et al (1996) Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol 55:481–490. doi:10.1097/00005072-199604000-00011
Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672. doi:10.1126/science.1154584
Tan CF, Piao YS, Hayashi S, Obata H, Umeda Y, Sato M et al (2004) Familial amyotrophic lateral sclerosis with bulbar onset and a novel Asp101Tyr Cu/Zn superoxide dismutase gene mutation. Acta Neuropathol 108:332–336. doi:10.1007/s00401-004-0893-4
Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A et al (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol 113:535–542. doi:10.1007/s00401-007-0206-9
Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB et al (2008) TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol 7:409–416. doi:10.1016/S1474-4422(08)70071-1
Yokoseki A, Shiga A, Tan C-F, Tagawa A, Kaneko H, Koyama A et al (2008) TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol 63:538–542. doi:10.1002/ana.21392
Zhang H, Tan CF, Mori F, Tanji K, Kakita A, Takahashi H et al (2008) TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 115:115–122. doi:10.1007/s00401-007-0285-7
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to K.W.) and a grant from the Research Committee on Neurodegenerative Diseases, Ministry of Health, Labor and Welfare, Japan (to H.T.). The authors wish to express their gratitude to M. Nakata for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, F., Tanji, K., Zhang, HX. et al. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 116, 193–203 (2008). https://doi.org/10.1007/s00401-008-0396-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0396-9