Abstract
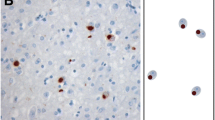
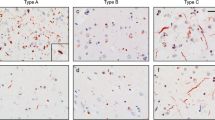
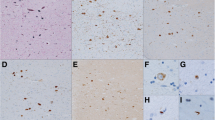
TDP-43 is a major component of ubiquitin-positive, tau-negative inclusions in amyotrophic lateral sclerosis (ALS), and frontotemporal lobar degeneration. We immunohistochemically examined the neostriatum from 14 cases of classic ALS (cALS), six cases of ALS with dementia (ALS-D), and 20 control subjects. TDP-43-positive, crescent or circular inclusions were found in neostriatal small neurons in 19 of 20 cases of ALS, but not in controls. Two types of inclusions were found in the large neurons: ubiquitin-positive, TDP-43-negative rod-like inclusions, and ubiquitin- and TDP-43-positive pleomorphic inclusions. The latter were specific to ALS; they were found in seven cases of cALS and in all of ALS-D. TDP-43-positive glial inclusions were also found in 12 cases of cALS and in all of ALS-D. These TDP-43-positive neuronal and glial inclusions were more numerous in ALS-D than cALS. In ALS-D, neuronal loss in the substantia nigra was found in all the cases, whereas mild gliosis without obvious neuronal loss was noted in the neostriaum in only two cases. These findings suggest that the neostriatum is also involved in the disease process of ALS with and without dementia.



Similar content being viewed by others
References
Al-Sarraj S, Maekawa S, Kibble M, Everall I, Leigh N (2002) Ubiquitin-only intraneuronal inclusion in the substantia nigra is a characteristic feature of motor neurone disease with dementia. Neuropathol Appl Neurobiol 28:120–128
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Anderson VER, Cairns NJ, Leigh PN (1995) Involvement of the amygdala, dentate and hippocampus in motor neuron disease. J Neurol Sci 129(Suppl):75–78
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Bigio EH, Johnson NA, Rademaker AW, Fung BB, Mesulam MM, Siddique N, Dellefave L, Caliendo J, Freeman S, Siddique T (2004) Neuronal ubiquitinated intranuclear inclusions in familial and non-familial frontotemporal dementia of the motor neuron disease type associated with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 63:801–811
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114:71–79
DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993
Goldman JE, Horoupian DS (1982) An immunocytochemical study of intraneuronal inclusions of the caudate and substantia nigra. Reaction with an anti-actin antiserum. Acta Neuropathol 58:300–302
Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K (2007) TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain 130:1386–1394
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H (2007) Appearance pattern of TDP-43 in Japanese frontotemporal lobar degeneration with ubiquitin-positive inclusions. Neurosci Lett 419:213–218
Hudson AJ (1981) Amyotrophic lateral sclerosis and its association with dementia, parkinsonism and other neurological disorders: a review. Brain 104:217–247
Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, Petersen RC, Davies P, Duara R, Graff-Radford NR, Uitti RJ, Rademakers R, Adamson J, Baker M, Hutton ML, Dickson DW (2007) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 66:142–151
Kato S, Oda M, Hayashi H, Kawata A, Shimizu T (1994) Participation of the limbic system and its associated areas in the dementia of amyotrophic lateral sclerosis. J Neurol Sci 126:62–69
Kawashima T, Kikuchi H, Takita M, Doh-ura K, Ogomori K, Oda M, Iwaki T (1998) Skein-like inclusions in the neostriatum from a case of amyotrophic lateral sclerosis with dementia. Acta Neuropathol 96:541–545
Kawashima T, Furuta A, Doh-ura K, Kikuchi H, Iwaki T (2000) Ubiquitin-immunoreactive skein-like inclusions in the neostriatum are not restricted to amyotrophic lateral sclerosis, but are rather aging-related structures. Acta Neuropathol 100:43–49
Kawashima T, Doh-ura K, Kikuchi H, Iwaki T (2001) Cognitive dysfunction in patients with amyotrophic lateral sclerosis is associated with spherical or crescent-shaped ubiquitinated intraneuronal inclusions in the parahippocampal gyrus and amygdale, but not in the neostriatum. Acta Neuropathol 102:467–472
Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ (2007) TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol 114:63–70
Leigh PN, Whitwell H, Garofalo O, Buller J, Swash M, Martin JE, Gallo J-M, Weller RO, Anderton BH (1991) Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain 114:775–788
Lowe J, Aldridge F, Lennox G, Doherty F, Jdederson D, Landon M, Mayer RJ (1989) Inclusion bodies in motor cortex and brainstem of patients with motor neuron disease are detected by immunocytochemical localization of ubiquitin. Neurosci Lett 105:7–13
Mackenzie IRA, Feldman H (2003) The relationship between extramotor ubiquitin-immunoreactive neuronal inclusions and dementia in motor neuron disease. Acta Neuropathol 105:98–102
Mackenzie IR, Feldman H (2004) Extrapyramidal features in patients with motor neuron disease and dementia; a clinicopathological correlative study. Acta Neuropathol 107:336–340
Mackenzie IRA, Feldman HH (2005) Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol 64:730–739
Mackenzie IR, Baker M, Pickering-Brown S, Hsiung GY, Lindholm C, Dwosh E, Gass J, Cannon A, Rademakers R, Hutton M, Feldman HH (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434
Massman PJ, Sims J, Cooke N, Haverkamp LJ, Appel V, Appel SH (1996) Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 61:450–455
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809
Nakano I (1993) Temporal lobe lesions in amyotrophic lateral sclerosis with or without dementia: a neuropathological study. Neuropathology 13:215–227
Neumann M, Sampathu DM, Kwong LK, Truax A, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM-Y (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS (2007) TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol 66:152–157
Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM, Clark CM, Grossman M, Miller BL, Trojanowski JQ, Lee VM (2007) TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol 66:177–183
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Okamoto K, Murakami N, Kusaka H, Yoshida M, Hashizume Y, Nakazato Y, Matsubara E, Hirai S (1992) Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol 239:426–430
Okamoto K (1998) Temporal lobe pathology in patients with amyotrophic lateral sclerosis. Neuropathology 18:222–227
Ota S, Tsuchiya K, Akiyama H (2005) “Forme fruste” of amyotrophic lateral sclerosis with dementia: a report of five autopsy cases without dementia and with ubiquitinated intraneuronal inclusions. Neuropathology 25:326–335
Oyanagi K, Takahashi H, Wakabayashi K, Ikuta F (1991) Large neurons in the neostriatum in Alzheimer’s disease and progressive supranuclear palsy: a topographic, histologic and ultrastructural investigation. Brain Res 544:221–226
Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T, Ikuta F, Oyanagi K, Takahashi H (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10–S17
Seelaar H, Schelhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, de Rijik MC, Rizzu P, Brummelhuis M X, van Doorn PA, Kamphorst W, Willemsen R, van Swieten JC (2007) TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain 130:1375–1385
Sieradzan KA, Mechan AO, Jones L, Wanker EE, Nukina N, Mann DM (1999) Huntington’s disease intranuclear inclusions contain truncated, ubiquitinated huntingtin protein. Exp Neurol 156:92–99
Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases, the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors (1994) El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 124(Suppl):96–107
Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol 113:535–542
Wakabayashi K, Piao YS, Hayashi S, Kakita A, Yamada M, Takahashi H (2001) Ubiquitinated neuronal inclusions in the neostriatum in patients with and amyotrophic lateral sclerosis with and without dementia—a study of 60 patients 31 to 87 years of age. Clin Neuropathol 20:47–52
Wightman G, Anderson VER, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Yoshida M (2004) Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology 24:87–102
Acknowledgments
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to K.W.), a grant from the Research Committee on Neurodegenerative Diseases, Ministry of Health, Labor, and Welfare, Japan (to H.T.), and the Karoji Memorial Fund for Medical Research (to K.T.). The authors wish to express their gratitude to M. Nakata for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Tan, CF., Mori, F. et al. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 115, 115–122 (2008). https://doi.org/10.1007/s00401-007-0285-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0285-7