Abstract
About 20% of all irrigated land is adversely affected by salinity hazards and therefore understanding plant defense mechanisms against salinity will have great impact on plant productivity. In the last decades, comprehension of salinity resistance at molecular level has been achieved through the identification of key genes encoding biomarker proteins underpinning salinity tolerance. Implication of the vacuolar transport systems in plant salinity tolerance is one example of these central mechanisms rendering tolerance to saline stress. One important organelle in plant cells is the central vacuole that plays pivotal multiple roles in cell functioning under normal and stress conditions. This review thus attempts to address different lines of evidence supporting the role of the vacuolar membrane transport systems in plant salinity tolerance. Vacuolar transport systems include Na+(K+)/H+ antiporters, V-ATPase, V-PPase, Ca2+/H+ exchangers, Ca2+-ATPase, ion channels, aquaporins, and ABC transporters. They contribute essentially in retaining a high cytosolic K+/Na+ ratio, K+ level, sequestrating Na+ and Cl− into vacuoles, as well as regulation of other salinity responsive pathways. However, little is known about the regulation and functions of some of the vacuolar transporters under salinity stress and therefore need more exploration and focus. Numerous studies demonstrated that the activities of the vacuolar transporters are upregulated in response to salinity stress, confirming their central roles in salinity tolerance mechanism. The second line of evidence is that manipulation of one of the genes encoding the vacuolar transport proteins results in some successful improvement of plant salinity tolerance. Therefore, transgene pyramiding of more than one gene for developing genotypes with better and strong salinity tolerance and productivity should gain more attention in future research. In addition, we should move step further and verify the experimental data obtained from either a greenhouse or controlled environment into field trials in order to support our claims.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
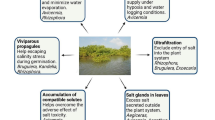
Salinity stress is a major threat to global agriculture as it reduces crop growth, development, and productivity. The elevated salinization of arable lands all over the world is anticipated to result in 50% loss by the year 2050 (FAO 2009). The deleterious effects of salinity stress on plants occur through imposing osmotic stress, specific ion toxicity, and oxidative stress due to increased production of reactive oxygen species (ROS) in plant cells (Mansour and Stadelmann 1994). These adverse effects occur in different timescales in roots and photosynthetic tissues: ion imbalance in leaves becomes critical only after many days or even weeks, while ionic effect and oxidative stress in roots occur in timescale of minutes to hours (Munns and Tester 2008). Figure 1 sums up these harmful effects of salinity stress. Plants cope with these hazard effects of the saline conditions by evolving different adaptive mechanisms. Salt-tolerant plants combat the osmotic action of salinity by producing organic osmolytes and/or absorbing ions from the soil (Mansour et al. 1993; Flowers et al. 2015). In order to overcome specific ion toxicity, tolerant plants adopt ion homeostasis by retaining essential elements (K+, Ca2+, Mg2+) and decreasing the toxic ones, Na+ and Cl− (Mansour 1995; Munns and Tester 2008; Flowers et al. 2015). Additionally, plants maintain ROS production under tight control by an efficient antioxidant defense system, enzymatic and nonenzymatic, leading to cellular redox homeostasis (Hasanuzzaman et al. 2012; Mansour et al. 2019). ROS at low concentration, however, can have positive implications because of the important signaling nature of some of their species as well as their central role in physiological and developmental processes throughout the plant life cycle (Mansour et al. 2019; Kimura et al. 2020; Eljebbawi et al. 2021). It is noteworthy that plant salinity tolerance has been early reported by Mansour and Stadelmann (1994) to be related to differences in the cytoplasmic characteristics that already exist before and after exposure to saline environment. Understanding the aforementioned tolerance mechanisms at physiological, biochemical, and molecular level (Fig. 2) certainly provides the advantage to develop approaches that can be utilized to mitigate salinity stress in salt-sensitive crops and produce salt-tolerant genotypes.
Physiological, biochemical, and molecular mechanisms rendering salinity tolerance to plants in response to saline conditions based on Mansour et al. (2021) (Color figure online)
Excess accumulation of Na+ ions is toxic for cell metabolism, disrupts plant nutrient balance by affecting the uptake of essential elements, and consequently results in high Na+/K+ ratio in the cytosol, which inhibits various processes such as vital enzyme reactions, protein synthesis, and photosynthesis under saline stress (Flowers et al. 2015; Shabala et al. 2016; Wang et al. 2017a). Plants should deal with this nutrient imbalance and ion toxicity to maintain plant growth and yield under saline conditions. Ion homeostasis with low Na+ and high K+ concentrations in the cytoplasm is therefore a key strategy to minimize the toxic ion harmful effects and to maintain normal metabolic and physiological processes under saline environments (Munns and Tester 2008; Flowers et al. 2015). Plants have to exclude Na+ and Cl− and/or sequester them into the vacuole to avoid their toxicity in the cytoplasm and maintain appropriate cellular levels of K+ and Ca2+ necessary for metabolic activities in response to high salinity. Actually, evidence indicates that salt-tolerant plant species have salinity tolerance mechanism operates at the root level via extrusion of toxic ions and restriction of excessive Na+ and Cl− transport to the leaves after salinity treatment (Huertas et al. 2012; Rubio et al. 2020; Zelm et al. 2020). The task of toxic ion exclusion is typically carried out by the plasma membrane (PM) transport proteins that exclude Na+ from the cytosol in exchange for H+ by the PM Na+/H+ antiporters (SOS1, Mansour 2014). Should salt exclusion is predominated, an energy burden puts on the plant in terms of biosynthesis of costly organic osmolytes required to ensure osmotic balance essential for water uptake and turgor maintenance under saline stress. Another tolerance trait is that Na+ can potentially be sequestered into vacuoles by sodium/proton antiporters (Na+/H+ antiporters, NHXs), which belong to the cation/proton antiporter (CPA1) family of transporters (Bassil et al. 2012; Jia et al. 2018; Al‑Harrasi et al. 2020). This results in Na+ detoxification of the cytoplasm. Both the PM Na+/H+ antiporters and tonoplast Na+/H+ antiporters are energy dependent driven by proton motive force generated by the PM and vacuolar proton pumps, respectively (Rubio et al. 2020; Zelm et al. 2020). Vacuolar Na+ sequestration has always been considered as one of the key components differentiating between sensitive and tolerant species/genotypes under saline environments (Wu et al. 2015). It is noteworthy that sequestrating Na+ into the vacuole not only reduces cytosolic Na+ toxicity but also gives an opportunity to use Na+ as a cheap osmoticum that participates in water retention needed for turgor and hence cell expansion under high salinity. Na+ sequestration strategy at the same time minimizes the high energy cost of organic osmolyte biosynthesis in response to saline stress (Mansour and Ali 2017). However, Na+ retention in vacuoles should be regulated tightly; otherwise, a futile Na+ cycle could be resulted costing plants a significant amount of energy (Shabala et al. 2020). This is because Na+ permeable slow vacuolar (SV) and fast vacuolar (FV) channels mediate the back-leak of Na+ into cytosol under salinity imposition (Assaha et al. 2017; Shabala et al. 2020). Na+ retention in vacuoles is therefore a crucial component of Na+ sequestration into the vacuole and salinity tissue tolerance. In addition, high-affinity K+ transporters (HKT, K+/H+ symporters, KUP/HAK/KT, KIR) play pivotal role in retaining a low cytosolic Na+ and a high cytosolic K+, and thus low Na+/K+ ratio in plants under salinity imposition (Qin et al. 2019). In support to their essential contribution to salinity tolerance mechanism, overexpression of the above transporter genes improves salinity tolerance in numerous crops (Zhang and Blumwald 2001; Bassil et al. 2012; Mansour 2014; Muchate et al. 2016; Jia et al. 2018; Qin et al. 2019; Al‑Harrasi et al. 2020). Genetic manipulation of these transporters therefore represents a feasible way to enhance ion homeostasis and salinity tolerance in crop plants under high salinity stress (Gupta and Huang 2014). As this review focuses on the data drawn from the tonoplast transport systems and their role in plant salinity resistance, readers are referred to the reviews by Aghaei and Komatsu (2013) and Mansour (2014) for the PM transport systems underpinning tolerance to saline conditions.
The plant vacuole is a membrane-bound organelle occupying up to 90% of the total cell volume. The vacuoles of plant cells are widely diverse in form, size and content and have multiple functions, including plant growth and development, storage of nutrients and metabolites, generation of turgor, protein degradation, and plant defense (Martinoia et al. 2007; Gao et al. 2015). The function of vacuole as a reservoir for ions and metabolites relies on tonoplast-localized transport proteins (Hedrich et al. 2015; 2018). For example, tonoplast sugar transporter allows accumulation of sugars against large concentration gradients by utilizing the proton gradient across the tonoplast driven by the vacuolar membrane proton pumps (Hedrich et al. 2015). The vacuolar membrane also plays an important role in the cytosolic Ca2+ homeostasis of plant cells, which occurs through the vacuolar Ca2+-ATPases (Liu et al. 2021) and vacuolar Ca2+/H+ exchange (CAX) mechanism (Dindas et al. 2021). The vacuole is thus a major source of Ca2+ for intracellular calcium signaling (Schönknecht 2013). As such, the vacuolar membrane or the tonoplast includes three major different types of transport systems that contribute to vacuolar Na+ sequestration: (a) tonoplast Na+/ H+ antiporter (NHX1) that pumps Na+ into the vacuole (Apse et al. 1999), (b) two types of vacuolar proton pumps (H+-ATPase, V-ATPase; and H+-pyrophosphatase, V-PPase) that are responsible for generating a proton gradient as a driving force for NHX1 operation (Muchate et al. 2016; Wang et al. 2020), and (c) SV and FV vacuolar channels that are permeable to Na+ and should be kept close to prevent Na+ back-leak from the vacuole (Bonales-Alatorre et al. 2013b; Koselski et al. 2019; Wu et al. 2019). In support, root vacuolar Na+ sequestration has been indicated to be highly efficient to account for the overall barley salinity tolerance than root Na+ exclusion under saline conditions (Wu et al. 2019). Other transport proteins in the vacuolar membrane are ion channels and receptors (Silva and Gerós 2009; Dindas et al. 2021). For instance, vacuolar membrane possesses the chloride channel proteins (CLC) that transport Cl− into the vacuole, contributing to the Cl− detoxification of the cytosol (Wei et al. 2019; Wu and Li 2019). In spite of the pivotal functions of the vacuolar transport systems in plant tolerance to high salinity, to our knowledge no comprehensive work has been done to synthesize a review addressing the types, roles, and gene transformation of these vacuolar transport proteins in contrasting plant species/genotypes in response to high salinity. Such information addressed in this article will help in focusing a key salinity tolerance trait that is central to improve crop tolerance and productivity under saline conditions. This review is therefore considered the newest developments in the field and is useful to researchers who are keen to advance the field and improve crop production under salinity stress. The updated knowledge of the vacuolar transport systems in response to salinity stress, their role in conferring tolerance to saline conditions, and their genes as molecular biomarkers are therefore pointed out and discussed in this review. The importance of these crucial molecular candidates lays on the fact that they could be potentially utilized in molecular breeding or genetic transformation programs to develop enhanced salt-tolerant crop genotypes that can withstand high salinity. Figure 3 summarizes that these transport systems are located at the tonoplast and largely contribute to plant tolerance to saline soil.
The tonoplast transport systems involved in ion homeostasis and contributed to salinity tolerance. 14-3-3 proteins, regulatory molecules that have the ability to bind diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors; ALMT aluminum-activated malate transporters, AQP aquaporin, CAX Ca2+/H+ antiporter, CLC chloride channel proteins, FV fast vacuolar channel, NHX vacuolar Na+/H+ antiporter, SCaBP Ca2+-binding protein (Ca2+ sensor), SOS2 salt overly sensitive 2 (a serine/threonine protein kinase), SV slow vacuolar channel, V-ATPase vacuolar H+-ATPase, VK vacuolar K+-selective channel, V-PPase vacuolar H+-pyrophosphatase. Red arrows indicate activation (Color figure online)
V-ATPase and Tolerance to Saline Conditions
Structure and Functions of V-ATPase
H+-ATPases in plant cells are widely distributed on intracellular membranes, including the PM, the chloroplast and mitochondrial membranes, and the vacuolar membrane (Wang et al. 2020, Fig. 3). V-ATPase composed of two kinds of domains: the peripheral V1 domain and the membrane-integrated V0 domain, which are assembled together in V1–V0 complex (Gupta et al. 2021). The peripheral V1 complex hydrolyzes the ATP molecule and is formed from eight different subunits (A–H), while the V0 domain pore complex facilitates proton transport through it and is made up of six different subunits (a, c, c', c'', d, e). All the V1–V0 subunits are encoded by one of the large gene families known as VHA genes (Gupta et al. 2021). Consequently, the V-ATPase is a multisubunit complex composed of at least 13 subunits, which have been reported to be involved in abiotic stress resistance besides its performing basic house-keeping functions (Wang et al. 2020).
The function of V-ATPase is usually analyzed through the study of its different subunits (Dietz et al. 2001; Adem et al. 2017; Wang et al. 2020; Table 1). Many studies showed that V-ATPase participates in the response of various plants to external salinity via providing the driving force for the vacuolar compartmentalization of Na+ (Gaxiola et al. 2007; Jiang et al. 2010; Bassil and Blumwald 2014). This Na+ sequestration is achieved by the vacuolar Na+/H+ antiporters, which essentially depend upon the activities of the V-ATPases and/or V-PPases (see next section) to provide ∆pH across the tonoplast (Muchate et al. 2016). Worthy of note is the vacuolar Na+ sequestration is not an energy-consuming process such as Na+ exclusion because the energy consumed in Na+ storage in the vacuole is much less than that required for biosynthesis of organic osmolytes utilized for osmotic adjustment and turgor maintenance (Yeo 2006; Saibi and Brini 2021). Besides eliminating the Na+ toxicity of the cytoplasm, vacuolar Na+ accumulation is an efficient cheap osmoticum for osmotic balance under salinity stress (Wang et al. 2020). As the function of V-ATPase is usually studied through its different subunits, the expression of different subunits of VHA gene in various tissues of Mesembryanthemum crystallinum (Low et al. 1996), Tamarix hispida (Gao et al. 2011; Wang et al. 2020), Arabidopsis thaliana (Zhao et al. 2009; Wang et al. 2011a; He et al. 2014; Wang et al. 2020), Broussonetia papyrifera (Zhang et al. 2012), Nicotiana tabacum (Xu et al. 2011), Oryza sativa (Abdul Kader et al. 2006; Baisakh et al. 2012), and Hordeum vulgare (Adem et al. 2017) was increased and significantly improved the response of the transgenic plants to salinity imposition. In these investigations, transgenic plants showed enhanced activities of V-ATPase and antioxidant enzymes, higher K+/Na+ ratio, Na+ accumulation, RWC, chlorophyll contents, and photosynthetic efficiency following salinity stress. In addition to VHA gene, overexpression of other transporters (e.g., HKT, NHX) involved in ion homeostasis in response to saline conditions is evident in the above works, suggesting that these subunit genes may activate other salt-responsive genes encoding these transporters and also confirm that more than one gene are needed to be pyramided in order to produce strong tolerance trait. Further, Peng et al. (2016) reported that the activity of V-ATPase was increased in leaves of salt-tolerant cotton genotype, compared with the sensitive one, after 24 h of salt shock; the effect was accompanied by enhanced expression of GhNHX1, GhVAP-c2, and GhVAP-c4. The results demonstrated that the salt-tolerant genotype had a greater ability to increase Na+ sequestration into the leaf vacuoles and further modulated ion homeostasis to cope with high salinity (Table 1). Similarly, gene expression of HvNHX1 and VHA was enhanced in shoots of barley salt-tolerant genotype, relative to the sensitive one, after salinity exposure leading to higher content of cytoplasmic K+ and K+/Na+ ratio (Yousefirad et al. 2018). Moreover, wild rice Oryza rufipogon as a superior salinity tolerant exhibited efficient leaf photosynthesis, less damage to leaf tissues (tissue tolerance), and higher tissue Na+ accumulation in response to salinity stress, which were associated with the upregulation of genes of VHA-c, HKT1;4 (contribute to Na+ exclusion and K+ retention) and NHX1 (participate in Na+ detoxification achieved by vacuolar sequestration) (Solis et al., 2021), altogether involved in ion transport and sequestration. Taken together, the data underline the pivotal role of the vacuolar ATPase subunits as an important factor supplying the energy required for the transport proteins that remove Na+ from the cytosol into the vacuole and hence improving salinity tolerance. These findings also strongly suggest these subunits genes for genetic engineering potentiality to enhance crop resistance to high salinity.
Expression and Activity of V-ATPase Under Salinity Stress
In response to salinity stress, activity, structure, expression, and quantity of V-ATPase are changed in various plants to adapt to environmental conditions (Barkla et al. 1995; Tsiantis et al. 1996; Qiu et al. 2007; Silva et al. 2010; Wang et al. 2020). These reports have therefore shown that transcript and protein levels of the V-ATPase are increased during salinity stress. For example, the activity of V-ATPase was promoted in both the roots and leaves of Broussonetia papyrifera after salinity treatment; increased V-ATPase activity has been observed to relate to de novo synthesis of tonoplast proteins (Zhang et al. 2012). Proteomic investigation in Salicornia europaea illustrated a significant increase in the abundance of V-ATPase subunit A under salinity and a correspondence between the inhibition of a gene encoding the A subunit of V-ATPase (SeVHA-A) and the decline in protein activity (Lv et al. 2017). The results indicate that the subunit A of SeVHA encoded by SeVHA-A gene participates in vacuolar Na+ sequestration through regulating V-ATPase and V-PPase activities and that post-transcriptional or post-translational regulation can influence the tolerant trait of halophytes. Another study also reported V-ATPase upregulation by salinity stress, which was correlated with the increase in protein content and co-expression between A and E V-ATPase subunits in salt-tolerant Vigna unguiculata (Sobreira et al. 2014).
Consistently, other studies revealed that salinity-mediated increase in V-ATPase activity was a result of the increase in protein expression in various plant species (Wang et al. 2001; Silva and Gerós 2009; Queiros et al. 2009; Krishnamurthy et al. 2014; Miranda et al. 2017; Jaarsma and de Boer 2018). In the study of Jaarsma and de Boer (2018), protein amounts of the two vacuolar proton pumps decreased in the potato salt-sensitive cultivar but remained unchanged in the tolerant one, indicating their participation in salinity tolerance of potato. Additionally, Wang et al. (2001) demonstrated that upregulation of V-ATPase was the main strategy of salinity tolerance in the halophyte Suaeda salsa, while V-PPase plays minor role after salinity treatment. In support to involvement of V-ATPase in salinity tolerance is the finding that H+ transport of salt-sensitive Kalanchoe daigremontiana was inhibited following salinity treatment (White and Smith 1989). One facet of NH4+-induced salinity tolerance in sorghum was the induction of V-ATPase activity which was associated with upregulation of SbVHA2 expression and not V-PPase (Miranda et al. 2017). Furthermore, the hydrolytic and H+ pumping activity of V-ATPase increased in the halophytes Suaeda salsa (Qiu et al. 2007), Mesembryanthemum crystallinum (Barkla et al. 1995), and suspension-cultured cells of Populus euphratica (Silva et al. 2010) treated with NaCl. Na+ accumulation in leaves was also associated with increased activity of V-ATPase up to 300 mM NaCl in halophyte Cakile maritime, assuming Na+ vacuolar sequestration by tonoplast Na+/H+ antiporter (Debez et al. 2006). In the aforementioned studies, these effects were consistent with the high activity and expression of Na+/H+ antiporters and proton pumps in the vacuoles as well as those in the PM in the roots, which results in efficient Na+ exclusion from the cell, counteracted net Na+ accumulation in the cytosol and thus prevented the loading of Na+ into the xylem sap avoiding its accumulation in the photosynthetic tissues. Similarly, V-ATPase and V-PPase (Fig. 3) activities were promoted in leaves of Nitraria tangutorum up to 300 mM NaCl (Liu et al. 2014), suggesting a pivotal role in providing the energy for stimulating Na+ vacuolar compartmentalization by NHX transporters in response to salinity. Accordingly, it is obvious that plant survival under saline conditions largely relies on the maintenance of V-ATPase activity/expression which contributes in lowering cytosolic Na+ levels and salinity tolerance. In addition, it is evident from the previous studies that several genes are modulated to confer tolerance to salinity stress.
Regulation of V-ATPase Activity Under Salinity Stress
Different mechanisms have been shown to regulate the vacuolar proton pumps during salinity stress and hence affect plant resistance to high salinity. First, Cl− accumulation in the vacuole by tonoplast CLC dissipates an inside-positive membrane potential and thus stimulate restoration of the electrochemical potential gradient by vacuolar ATPase and PPase activities (Wu and Li 2019). It assumes therefore that elevated Cl− accumulation in the vacuole enhances the activities of vacuolar proton pumps to restore the pH gradient across the tonoplast between the vacuole and cytosol, resulting in Na+ detoxification and osmotic homeostasis. In support, Silva et al. (2010) illustrated enhanced ability of V-PPase to create an H+ gradient in the presence of high Cl− in the vacuole. Also, high amount of Cl− was found in the vacuole than in the cytoplasm and cell wall in P. euphratica (Chen et al. 2002; Gu et al. 2004), suggesting vacuolar Cl− sequestration, which is likely stimulating vacuolar pumps to re-establish the depolarized proton gradient necessary for Na+ compartmentalization.
Another regulation mechanism of V-ATPase activity is through post-translational modification (phosphorylation), by interaction with regulatory proteins (SOS2, WNK8, CDPK, 14-3-3 proteins, Fig. 3) or metabolic enzymes (enolase, aldolase). Post-translational regulation was proposed to be involved in salinity-mediated changes of V-ATPase activity in potato cell cultures (Queirós et al. 2009). In addition, SOS2 has been shown to regulate V-ATPase activity in Arabidopsis via direct interaction of the SOS2 protein with V-ATPase subunit B resulting in its activation (Batelli et al. 2007, Fig. 3). Also, Klychnikov et al. (2007) found out that 14-3-3 proteins interact with some of the subunits (VHA-A) in a phosphorylation-dependent way in Hordeum vulgare and activates the V-ATPase. Similarly, Neuhaus and Trentmann (2014) indicate that binding 14-3-3 proteins with V-ATPase regulates the pumping activity of V-ATPase. Other possible mechanism for regulation of the V-ATPase include in vitro evidence that WNK8, a member of the Arabidopsis WNK family of protein kinases, binds to and phosphorylates VHA-C of the V-ATPase promoting its activity (Hong-Hermesdorf et al. 2006). Furthermore, salt-treated Mesembryanthemum crystallinum plants revealed tonoplast association of glycolytic enzymes aldolase and enolase with subunits of the vacuolar V-ATPase, which was showed to may not only channel ATP to the V-ATPase but also directly upregulate H+ pump activity (Barkala et al. 2009). Moreover, one salinity tolerance feature showed in salt-tolerant soybean cultivar is its ability to generate more ATP via NDH (NADPH dehydrogenase)-dependent cyclic electron flow; these extra ATPs are consumed by V-ATPase to produce the proton motive force needed for Na+ compartmentation in the vacuole by Na+/H+ antiporter under salinity stress (He et al. 2015). This research pointing out the essentiality of V-ATPase in the sequestration of Na+ (K+) into the vacuole as well as the importance of this photosynthetic process in generating the ATP to energize ATP-dependent processes (i.e., Na+ (K+)/H+ exchanges), and thus impacting Na+ homeostasis and salinity tolerance. Interestingly, a loss of coupling efficiency between ATP hydrolysis and proton transport by V-ATPase has been reported at high ATP concentrations (Sobreira et al. 2014), indicative of an important way of regulating V-ATPase activity.
One further regulatory mechanism that might be involved in V-ATPase-modulated activity is lipid microenvironment surrounding the tonoplast transport proteins (Zhang et al. 2015b). For instance, differences in V-ATPase activity (E subunit), expression of V-ATPase protein, and lipid composition of vacuolar membrane contributed to differential response of two shrub willow clones to salinity stress (Zhang et al. 2018). Salinity-induced lipid peroxidation of vacuolar membrane lipids caused inhibition of V-ATPase activity in the leaves of the two shrub willow clones, whereas salt-adapted clone exhibited increased transcript levels of V-ATPase E subunit in roots (relative to sensitive clone) resulting in elevated V-ATPase activity and hence facilitating transport of excessive Na+ into the vacuole. Also, reduced tonoplast fluidity owing to increased degree of fatty acid saturation was another factor to slow down Na+ leakiness from the vacuole in salt-adapted clone (Zhang et al. 2018). It seems that lipid peroxidation reached a level inhibiting V-ATPase activity, but not injurious to other physiological processes in the leaf cells of both clones and appears that differential salinity tolerance resides in the roots of the salt-tolerant clone. That is, differential V-ATPase activity in the two shrub willow clones is modulated by its protein level and lipid microenvironment of the tonoplast in the root cells. Taken together, V- ATPase has been demonstrated to be regulated by diverse mechanisms, which impact V-ATPase potentiality to provide a driving force for Na+/H+ antiporter sequestration of Na+ into the vacuole under salinity stress. Because of the intricate nature of V-ATPase (relative to V-PPase), its expression regulation under salinity is very complex because of the previous regulatory agents as well as its various subunits. How these subunits coordinate and act together on improving holoenzyme activity is not fully known. However, the central role of these mechanisms as a hub to activate the protein that provides energy for others to remove Na+ from the cytosol into the vacuole is evident and cannot be excluded.
V-PPase and Tolerance to Saline Conditions
Functions and Activity of V-PPase During Salinity Stress
The electrogenic H+ pump V-PPase (Fig. 3) is an essential energizer of the vacuolar membrane of plant cells. In addition to V-PPase major roles in abiotic stresses, other functions have been reported such as maintaining cellular PPi homeostasis, heterotrophic growth, increased auxin transport, and sucrose transport from source to sink tissues (Schilling et al. 2014). Generally, V-PPase activity is high in young tissues, whereas V-ATPase activity is relatively constant during growth and maturation (Silva and Gerós 2009). The role of V-PPase in plant response and tolerance to salinity imposition has been extensively demonstrated in various plant species (Table 1). For example, V-PPase activity has been increased in NaCl-adapted cells of Acer pseudoplatanus and NaCl-treated Daucus carota cells over control cells (Barkla and Pantoja 1996). NaCl-adapted cells of Solanum tuberosum also showed higher V-PPase and V-ATPase activities as well as increased tonoplast Na+/H+ antiport activity relative to unadapted cells under NaCl stress (Queiros et al. 2009). The increased activity of the vacuolar pumps is essential to provide the driving force for the operation of Na+/H+ antiport NHX activity in response to salinity, which results in sequestration of excess Na+ into the vacuole. In the same trend, greater induction of tonoplast V-ATPase and V-PPase activities in salt-tolerant rice line than in the sensitive line was correlated with stronger activation of the tonoplast Na+/H+ antiporter in this tolerant line in response to salinity (Pons et al. 2011). In the last work, tonoplast proton pumps and antiporters showed a more rapid and stronger response to salinity than those in the PM, suggesting tonoplast proton pumps implication in salt stress signaling. Additionally, NaCl treatment led to an increase in V-PPase activity in the roots of Broussonetia papyrifera (Zhang et al. 2012). Also, V-PPase was upregulated by salinity stress, which was correlated with the increase in protein content in salt-tolerant Vigna unguiculata (Sobreira et al. 2014). Martínez-Alcántara et al. (2015) demonstrated that higher root Na+ concentration (vacuolar Na+) in salt-tolerant trifoliate orange genotype, relative to the sensitive genotype, permits lower allocation of Na+ in the shoots, which results in an enhanced retrieval of Na+ from xylem stream and an impaired translocation to the shoot tissues. This response was a consequence of enhanced activity of the root tonoplast V-ATPase and V-PPase together with higher transcriptional levels of NHX1 found in roots and shoots of the salt-tolerant genotype as well as the overexpression of SOS1 (in roots) and HKT1 (in roots and shoots). The work reveals the preferential sequestration into vacuole of retrieved Na+ from xylem mainly in roots and thus averting the toxic effects of Na+ and maintaining cell osmotic equilibrium. Parks et al. (2002) also report that in salt halophyte Salicornia bigelovii, efficient vacuolar sequestration of Na+ by vacuolar Na+/H+ antiporter is associated with increased V-PPase activity and its protein accumulation, suggesting a role of both transport systems in salinity tolerance. It seems that providing more driving force for Na+/H+ antiporters under salinity stress is most likely the result of the activities of both proton pumps of the tonoplast (Fig. 3). However, without salinity stress, plants exhibited negative feedback on cell viability and thus plants need to regulate the V-PPase pump activity during normal growth.
V-PPase Genes and Response to High Salinity
Following salinity treatment, the wheat V-PPase genes (TaVP1 and TaVP2) showed induced expression (Table 1, Wang et al. 2009), pointing out to their involvement in coping with saline conditions. Guo et al. (2019) similarly illustrated that NHX1 and V-PPase were among the salt-responsive genes that play vital roles in Na+ sequestration in salt bladders of Atriplex canescens under NaCl treatment. Based on their role in response to salinity, these tonoplast transporters are most likely related to salinity tolerance. Furthermore, one of the salt-tolerant genes that its expression was higher in salt-tolerant sorghum genotype during salinity stress was H+-PPase (Punia et al. 2020), which indicates the contribution of this tonoplast proton pump in salinity tolerance of sorghum. Moreover, the increases in the transcription level of V-PPase result in improving tolerance to salinity in transgenic alfalfa, which accumulates more Na+, K+, and Ca2+ in leaves and roots as well as higher photosynthesis capacity and lesser cell membrane damage under salinity stress (Bao et al., 2008). These findings suggest that V-PPase genes, in addition to others, are important regulators in the tolerance of plants to salinity stress and can serve as a useful genetic resource to improve plant tolerance to high salinity. The above studies clearly infer that several genes act cooperatively to minimize the salinity hazards and enhance adaptation to saline conditions. The essentiality of V-PPase genes in plant adaptation to saline soil is also confirmed by studies which demonstrated that overexpression of V-PPase genes improved salinity tolerance of several plant species in response to salinity stress (next section). This is because overexpression of the V-PPase would enhance the proton pumping activity at vacuolar membrane and thus permit to accumulate more Na+ in vacuoles due to activity of vacuolar Na+/H+ antiporters.
Overexpression of V-PPase-Encoding Genes Improved Salinity Tolerance
Genetic manipulation of V-PPase expression in different species has great potentiality for improvement of plant salinity resistance. The genetic manipulation choice of V-PPase is certainly explained by a single gene required for the protein, while the other tonoplast V-ATPase is composed of several subunits and needs correct overexpression of several genes (Silva and Gerós 2009). For instance, overexpression of AVP1 in Arabidopsis, tomato, cotton, and rice enhances plant performance under saline conditions (Pasapula et al. 2011). In this work, AVP1 expressing cotton showed more vigorous growth, improved salinity tolerance, and greater fiber yield than wild-type plants in saline soil-grown greenhouse conditions. Transgenic plants showed higher proton electrochemical gradient facilitating sequestration of ions and sugars into the vacuole, which reduces water potential and resulting in increased salinity tolerance when compared with wild-type plants. Consistently, introgression of SbVPPase in finger millet enhanced the transgenic performance, increased Na+ and K+ contents, proline and chlorophyll levels, yield parameters, and antioxidant enzyme activities, and reduced lipid peroxidation under 200 mM NaCl stress (Anjaneyulu et al. 2014), demonstrating the positive effects of SbVPPase expression in enhanced salinity tolerance by facilitating efficient sequestration of excess Na+ ions into vacuoles and scavenging ROS. Further, transgenic Arabidopsis-overexpressing V-PPase accumulated more Na+ and K+ in the leaves and had higher V-PPase activity and water retention compared with wild plants (Gaxiola et al. 2001). Similarly, SeVP1 or SeVP2 transgenic Arabidopsis and wheat plants outperformed the wild types when grown under salinity and low nitrogen; this impact was shown in maintenance of higher K+/Na+ ratio in leaves, soluble sugars in shoots and roots, increased NO3− uptake, and vacuolar nitrate efflux (Lv et al. 2015). The results of Lv et al. (2015) suggest that upregulation of V-PPase favors the transport of photosynthates to root, which promotes root growth and integrates N and carbon metabolism in plant. Chen et al. (2015) also report that overexpression of ZmVP1 in Arabidopsis thaliana resulted in more vigorous growth under salinity imposition by accumulating more Na+ and K+ in the leaves, had higher activities of V-ATPase and V-PPase, and showed higher relative gene expression levels of AtNHX1, AtLEA, AtP5CS, AtMn-SOD, and AtAPX1. This work demonstrates that V-PPase contributes to salinity tolerance through regulating Na+ compartmentation into the vacuole, K+ assimilation, osmotic regulation, and antioxidant response, suggesting that overexpression of ZmVP1 regulates other salt-inducible genes.
Other works similarly showed that overexpression of Arabidopsis V-PPase gene AVP1 improved salinity tolerance in transgenic peanut plants (Qin et al. 2013), barley under greenhouse or saline soil conditions (Schilling et al. 2014), alfalfa (Bao et al. 2009; Su et al. 2019), and Lotus corniculatus (Cheng et al. 2011). These studies evidenced that AVP1 gene overexpression energized the vacuolar membrane for Na+ sequestration into the vacuole, which enhanced osmotic balance of cells and reduced the damage of excess Na+ in the cytosol. In addition, transgenic Nicotiana benthamiana plants constitutively overexpressing NbVHP were shown to have improved salinity tolerance after salinity treatment, as this vacuolar proton pump generates the pH gradient necessary for vacuolar proton-coupled Na+ sequestration (Graus et al. 2018). Transgenic creeping bentgrass plants overexpressing AVP1 exhibited improved resistance to salinity than wild-type plants; the transgenic improved performance was associated with higher biomass production, proline, RWC, concentrations of Na+, K+, Cl−, and total phosphorus in root tissues, and lower solute leakage in leaf tissues (Li et al. 2010). Another study revealed that transgenic cotton plants overexpressing TsVP, a V-PPase gene from Thellungiella halophila, exhibit a significantly elevated capacity to resist salinity stress compared with the wild type (Lv et al. 2008). Li et al. (2014a) also indicated that when wheat TaVP gene was overexpressed in tobacco, plant growth was improved under high salinity, which was found to be closely related to elevated V-PPase activities in the tonoplast, enlarged root systems, high dry mass, photosynthetic efficiencies, antioxidant enzyme activities, and soluble carbohydrate concentrations. In the previous works, higher Na+ accumulation in the roots and aerial tissues of transgenic plants than in wild-type plants under saline conditions points out to the indirect role of increased V-PPase activity in Na+ sequestration into the vacuole in order to avert excess Na+ toxicity of the cytoplasm. Few studies examined the exact function of V-PPase in improving salinity tolerance of trees or woody plants. For instance, overexpression of PtVP1.1 in poplar led to more vigorous growth of transgenic plants in the presence of 150 mM NaCl; transgenic plants exhibited higher V-PPase hydrolytic activity, decreased Na+ and increased K+ accumulation in the leaves, and higher Na+ efflux in the roots relative to wild type after NaCl treatment (Yang et al. 2015). Enhanced salinity tolerance in lines overexpressing VP1 suggest that VP1 might be an effective gene for salinity resistance improvement via genetic engineering approaches.
It is interesting to note that co-expression of genes encoding V-PPase and tonoplast Na+/H+ antiporter produced transgenic lines with stronger salinity tolerance than single gene transformants after salt exposure. For example, co-expression of AVP1 (from Arabidopsis) and PgNHX1 (from Pennisetum glaucum) conferred an increased fruit production and enhanced salinity tolerance to the transformed tomato compared with the AVP1 and PgNHX1 single gene transgenic plants and the wild type when grown under 200 mM NaCl (Bhaskaran and Savithramma 2011). The transgenic line co-expressing AVP1 and PgNHX1 retained much more chlorophyll, proline, and high Na+ content (sequestered into the vacuole) in response to salinity than single gene transformants. Also, transgenic rice co-expressing the Suaeda salsa SsNHX1 and Arabidopsis AVP1 (V-PPase) showed enhanced salinity tolerance during 300 mM NaCl stress by increasing V-PPase hydrolytic activity, K+/Na+ ratio, photosynthesis, and reducing H2O2 content, more so than the single SsNHX1(Zhao et al. 2006). Similarly, Shen et al. (2015) have also reported that transgenic cotton plants expressing the Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 and AVP1 produced significantly higher biomass compared with wild type and single transgenic plants under saline conditions. In addition, expression of HVP1 is coordinated with that of HvNHX1 in barley roots in response to salinity stress, and their co-expression greatly increase tolerance of barley more than in the case of the overexpression of either of them (Fukuda et al. 2004). Another research revealed that transgene alfalfa lines showed improved salinity and saline–alkali resistance by co-expression of the NHX1 and V-PPase genes (Liu et al. 2013). In the last research, ScVP/ScNHX1-co-expressing alfalfa plants accumulated more Na+ in leaves and roots under 300 mM NaCl with 100 mM NaHCO3; the results are clear evidence of the high potentiality of co-expression of multiple effective genes in conferring better and strong resistance to saline environments. Moreover, two genes from wheat, TNHXS1 (vacuolar Na+/H+ antiporter) and TVP1 (H+-pyrophosphatase), were introduced into tobacco genome resulted in the induction of the activities of the two important vacuolar ion transporters leading to high K+ and low Na+ levels in the leaf tissue, which improved salinity tolerance of transgenic tobacco under high salinity (Gouiaa et al. 2012). The results of this investigation consistently illustrated that when the two important genes were co-expressed, the performance of the transgenic tobacco was much better than when either the single gene was transformed under salinity stress. A work carried out by Sathee et al. (2015) illustrated that expression levels of NHX1 and VP1 were higher in leaves and roots of salt-tolerant wheat genotypes under 200 mM NaCl stress, indicative of their participation in ion homeostasis under salinity stress. Therefore, pyramided transgenic plants should be focused in the future research as a promising strategy to genetically engineer new cultivars of agriculturally important crops that are adapted to severe salinity.
V-PPase-Negative Correlation with Salinity Tolerance
Despite the above promising findings, contrasting results have been published concerning salinity responses of the V-PPase, which appears to be plant species, treatment type, and duration dependent in some studies. These discrepancies have been reviewed by Silva and Gerós (2009). For instance, V-PPase activity of suspension-cultured cells of Populus euphratica was reduced when treated with NaCl (Silva et al. 2010). Additionally, salinity stress reduced the V-ATPase and the V- PPase activity in two potato cultivars contrasting in their salinity tolerance; the decline in H+ pump activity was more severe in the salt-sensitive cultivar (Jaarsma and de Boer 2018). In this study, protein amounts of the two vacuolar H+ pumps decreased in the salt-sensitive cultivar but remained unchanged in the tolerant one, which may explain the reduced V-PPase activity found in salt sensitive after salinity stress. It appears that the higher resistance of the salt-tolerant cultivar largely depends on V-PPase activity to energize the observed greater Na+/H+ exchange activity across the tonoplast under salinity. A recent study unexpectedly also showed that the higher expression levels of both HvNHX1 tonoplast Na+/H+ antiporters and HvVP1 H+-pumps in salt-sensitive genotypes of barley (relative to tolerant ones) are unable to sequester Na+ in root vacuoles because of Na+ back-leak into the cytosol and existence of a futile Na+ cycle at the tonoplast (Wu et al. 2019). The study also illustrated that the PM Na+/H+ antiporter-mediated Na+ extrusion from the root plays a minor role in the overall salinity tolerance in barley. The results are further supporting that root vacuolar Na+ sequestration but not exclusion from uptake plays the main role in barley salinity tolerance. In order to prevent back-leak into the cytosol and forming a futile Na+ cycle at the tonoplast, the SV and FV channels should be tightly regulated and hence they are considered an important component of adaptation to high salinity (Wu et al. 2019). The authors also concluded that increased transcript levels of HvNHX1 and HvVP1 per se are not sufficient for achieving efficient vacuolar Na+ sequestration, rather post-translational regulations and/or inability of sensitive varieties to control the permeability of FV or SV channels seem to cause such discrepancy. Despite these few inconsistencies, V-PPase activity and overexpression have proven to be essential in improving salinity tolerance in many species/cultivars.
Vacuolar Ca2+-ATPase and Tolerance to Saline Conditions
In terms of energy cost of plant salinity tolerance, other tonoplast pumps should be considered. Besides vacuolar H+-ATPase and V-PPase, the Ca2+-ATPase (Fig. 3) should be therefore counted in (Shabala et al. 2020; Liu et al. 2021). In addition, of special interest is the fact that salinity-induced injurious effects have been mitigated by exogenous Ca2+ (Mansour 1995; Wang et al. 2017b). As for the function of this tonoplast pump, Ca2+-ATPase and Ca2+/H+ exchangers (CAXs, next section) move Ca2+ against its electrochemical potential gradient from the cytosol into the vacuole (Fig. 3) and hence Ca2+ transport into the large central vacuole is an essential part of cytosolic Ca2+ homeostasis (Schönknecht 2013). In addition, Ca2+-ATPase produces the electrochemical potential gradient that is required for operation of ATP-driven Ca2+/H+ exchanger. Plant growth and development, mineral nutrition and toxicity, stress signaling, salt stress response, and tolerance are also proposed physiological roles of Ca2+-ATPases (Bonza and De Michelis, 2010). Furthermore, one of the major hallmarks of early stress responses is a rapid influx of Ca2+ into the cytosol immediately after the stress recognition (Hilleary et al. 2020) and thus different vacuolar Ca2+ pumps and Ca2+ channels/transporters may contribute to Ca2+ signaling in plant cells under stress conditions (Schönknecht 2013). It can be therefore assumed that the tonoplast Ca2+-ATPase is an integral component of plant stress signaling and tolerance. In support to our proposal, it is reported that the subsequent change in cytosolic Ca2+ level is thought to play a role in triggering downstream responses, what so-called calcium signature (Schönknecht 2013; Hilleary et al. 2020). The authors also indicated that Ca2+-ATPase is actually involved in shaping the cellular Ca2+ dynamics during the triggering of the defense response network. The importance of these Ca2+ pumps come from the fact that cytosolic Ca2+ oscillations have been shown to depend on Ca2+ influx into the cytosol and Ca2+ removal from the cytosol by Ca2+ pumps (Schönknecht 2013).
The contribution of the vacuolar Ca2+-ATPase in adaptation to saline environments is provided by the finding that when the moss Physcomitrella patens exposed to 250 mM NaCl, cytosolic Ca2+ transients were more than twofold enhanced and dramatically prolonged, whereas the knockout plants displayed reduced expression of a stress-responsive Ca2+-ATPase gene (PCA1) and decreased salinity tolerance (Qudeimat et al. 2008). The work suggests that disturbance of a stress-associated signaling pathway thereby evidenced the role that Ca2+-ATPase plays in Ca2+-mediated signaling events under salinity (Table 1). Additionally, salinity stress-induced elevation in cytosolic Ca2+ and the new cytosolic Ca2+ status has been reported to be regulated by the tonoplast Ca2+/H+ antiporter and Ca2+-ATPase (Seifikalhor et al. 2019), suggesting that both transporters via their regulation of cytosolic Ca2+ largely participate in triggering salinity stress responses. However, it was shown that maize seedlings exposed to 100 mM NaCl rather enhanced hydrolytic than transport activity of Ca2+-ATPases in vacuolar membrane of root cells (Rudnytska and Palladina 2017). It is likely that formation of a higher vacuolar Ca2+ gradient leads to a feedback inhibition of the pump transport activity. Although Ca2+-ATPase of the vacuolar membrane may play important role in formation of cell response to salinity, its function in plant tolerance to saline environment has not been yet established and little information is available and therefore remains to be further studied and elucidated.
Vacuolar Ca2+/H+ Exchangers (CAXs) and Tolerance to Saline Conditions
Another vacuolar transporter that might have a role in plant response and tolerance to saline soil is Ca2+/H+ exchangers (CAXs, Fig. 3). These Ca2+/H+ antiporters have been identified in tonoplast membrane vesicles or vacuoles, and their activity has been detected in various plant species (Maeshima 2001). The activity of Ca2+/H+ exchangers is directly upregulated by cytosolic Ca2+ elevation mediated by ion channels (Demidchik et al. 2018). CAXs proteins belong to the multigene family of cation/H+ exchangers. However, Maeshima (2001) reported that the amount of CAXs proteins is very low compared with the vacuolar proton pumps and TIPs, making it difficult to recognize the antiporter protein in SDS–polyacrylamide gels. CAXs mediate Ca2+ transport which is always associated with the counter transport of another cation (usually H+ or Na+), which is fueled by the proton gradient produced via the V-ATPase and V-PPase (Liu et al. 2021). Compared with the high affinity and low capacity of the tonoplast Ca2+-ATPase pumps, CAXs have a low affinity and high transport capacity for Ca2+. Both CAXs and Ca2+-ATPases contribute to maintaining the Ca2+ concentration gradient between cell compartments, but in different modes, maybe in the way of fine tune and coarse tune, respectively (Liu et al. 2021). Similar to Ca2+-ATPase, CAXs are therefore critical for Ca2+-mediated phenomena in plants. Owing to their important role in regulating both intracellular and apoplastic pH, CAXs are crucial in a broad range of developmental processes (Cho et al. 2012). As such, CAXs are implicated in an increasing range of cellular and physiological functions, of specific importance is the role of CAXs in cell-specific calcium storage and defense responses (Demidchik et al. 2018). Several studies report that both CAXs expression levels and activity are highly cell type specific and modulated by various stresses (Punshon et al. 2012; Wang et al. 2016b; Demidchik et al. 2018). For example, in halophytic plants, CAXs have been recruited to play a role in salinity tolerance, in some cases as a modulator of cytosolic Ca2+ signaling, but in others CAXs acting as a pH regulator. Hocking et al. (2017) reported that Ca2+/H+ antiporters play a role in intracellular Ca2+ homeostasis, as they demonstrated that the interactions between CAX proteins contribute to the functioning of stomata because stomata were more closed in cax1-1, cax3-1, and cax1-1/cax3-1 loss-of-function mutants due to an inability to buffer Ca2+ effectively. It is thus hypothesized that the formation of CAX1–CAX3 complexes may occur in the mesophyll to affect intracellular Ca2+ signaling during defense responses. Consistently, apoplastic-free Ca2+ was threefold greater in cax1/cax3 than in wild-type plants (Conn et al. 2011), suggesting CAX1 as a key regulator of apoplastic Ca2+ through its compartmentation into mesophyll vacuoles. Moreover, modulation of CAX activities could occur by interaction with SOS2, which activates the tonoplast CAX antiporters (Pardo and Rubio 2011; Neuhaus and Trentmann 2014; Demidchik et al. 2018). It is interesting to mention that SOS2 regulates Na+ and also Ca2+ uptake into the vacuole (Fig. 3), which might imply that cellular Na+ and Ca2+ homeostasis are interconnected systems. Possible abiotic stress tolerance functions of CAXs make them attractive targets for biotechnology to provide salinity-tolerant genotypes. Despite the knowledge of CAX gene family and possible promising abiotic stress tolerance functions of CAXs that make them attractive targets for biotechnology, the role of Ca2+/H+ exchangers in salinity tolerance are emerging and still at their first steps and certainly requires more detailed research and elucidation.
Vacuolar Na+/H+ Antiporter (NHX) and Tolerance to Saline Conditions
Structure, Functions, and Localization of NHXs
According to their subcellular localization, the intracellular NHX antiporters are divided into two main groups, including the tonoplast-localized NHXs (class I) and the endosome localized NHXs, class II (Jia et al. 2018). Class I NHX catalyzes Na+/H+ and K+/H+ exchange, while class II NHX antiporter affects the accumulation of K+ but not Na+ in intracellular compartments (Pardo and Rubio 2011; Shabala et al. 2020; Saibi and Brini 2021). NHX proteins are of monovalent cation/proton antiporter family (CPA, Gupta et al. 2021). Among the six intracellular NHX isoforms identified in Arabidopsis, four distinct NHX isoforms named AtNHX1 to AtNHX4 locate to the tonoplast where AtNHX1 and AtNHX2 are the most predominant ones (Jia et al. 2018). Class II isoforms (AtNHX5 and AtNHX6, and the tomato LeNHX2) occur on endosomal membranes of the Golgi, trans-Golgi network, and pre-vacuolar compartment (Jiang et al. 2010; Bassil et al. 2012; Assaha et al. 2017). A recent work by Bassil et al. (2019) indicated that AtNHX1 and AtNHX2 are the main contributors to vacuolar pH, K+, and Na+ uptake, while NHX4 may have a high affinity for K+ and is less significant in Na+ uptake, contrasting with NHX3 which is involved in vacuolar Na+ transport. It is now clear that the main role of NHXs under normal conditions is a K+/H+ antiporter, with no concurrent transport of Na+, while Na+ transport mediated by NHXs would only occur under conditions of high Na+ concentration (Assaha et al. 2017). It is also reported that AtNHX1, in addition to its role as a Na+ (K+)/H+ antiporter, plays a significant role in intracellular vesicular trafficking, regulation of pH, transcription, K+/Na+ ratio and ion homeostasis, reduced oxidative damage, and salinity tolerance (Martinoia et al. 2007; Bassil et al. 2011, 2012; Wang et al. 2016a; Assaha et al. 2017; Zeng et al. 2018; Wu et al. 2018). Figure 4 sums up these different functions of NHX transporters that enable plants to combat the harmful effects of saline soil. In response to saline conditions, these NHX antiporters avoid cytoplasmic Na+ toxicity and participate in retaining cytosolic K+/Na+ ratio. Reviewing the literature, it turns out that NHX1 is the most studied intracellular transport protein under high salinity, and many studies therefore report vacuolar Na+/H+ antiporter NHX1 as one of the most important factors implicated in plant salinity tolerance. It is obvious that NHX antiporters are localized in various intracellular membranes playing crucial role in ion homeostasis under saline environments.
NHX Genes and Response to Salinity Stress
Gaxiola et al. (1999) report that AtNHX1 gene from Arabidopsis, encoding a vacuolar Na+/H+ antiporter, mediates compartmentalization of Na+ from cytosol into the vacuole and thus significantly contributes to salinity tolerance under saline conditions. Also, NaCl stress increased the mRNA level of OsNHX1, 2, 3, and 5 in rice (Fukuda et al. 2011), which indicates NHX important role in ion homeostasis and regulation under salinity stress. A recent study similarly revealed that salinity treatment induced OsNHX1 gene expression in the root and leaf tissue of salt-tolerant rice cultivar greater than in the sensitive cultivar (Theerawitaya et al. 2020, Table 2). As Na+ content was largely increased in the root tissues of salt-tolerant seedlings, overexpression of OsNHX1 gene most probably regulated the translocation of Na+ from root to leaf tissues and compartmentation of Na+ into vacuoles, thereby maintaining the photosynthetic abilities of this tolerant cultivar. Additionally, roots of salt-tolerant pistachio genotype, compared with sensitive one, showed a higher Na+/K+ ratio and higher levels of NHX1 transcript than the leaves (Rahneshan et al. 2018), indicating protection of the photosynthetic processes in the leaves from the deleterious effects of Na+ as well as preferential sequestration of Na+ in the root vacuoles. This Na+ compartmentation prevents the toxic effects of Na+ and simultaneously retains the osmotic equilibrium of root cells. This osmotic equilibrium is needed for generating the water potential gradient between the soil and the plant root; this water potential gradient maintains water absorption even under salinity-induced soil water deficit. Further, transgenic poplar plants expressing either AtNHX1 or AtNHX3 gene exhibited increased resistance to salinity stress by accumulating more Na+ and K+ in the vacuoles of the leaf cells in presence of 100 mM NaCl (Yang et al. 2017), indicating that constitutive expression of either of the two genes enhanced the Na+/H+ or K+/H+ exchange activity.
Research conducted by Cosentino et al. (2010) showed that expression of Na+⁄H+ antiporters in Mesembryanthemum crystallinum leaves and roots exhibited varied regulation under salinity stress; vacuolar McNHX1 was one of the three transporters (besides McSOS1 and McNhaD) playing a role in Na+ compartmentation in leaves, but not in roots, during plant adaptation to high salinity. It seems that McNHX1 is most likely responsible for Na+ accumulation in vacuolar and pre-vacuolar compartments of leaf mesophyll cells. It is also speculated that root cells adopted Na+ extrusion strategy (i.e., by McSOS1), whereas Na+ accumulation in chloroplasts is achieved by McNhaD. Recently, introduction of wheat TaNHX2 gene into the eggplant improved growth performance, Na+ and K+ contents of leaves and roots, leaf RWC, chlorophyll and proline contents, photosynthetic efficiency, transpiration rate, and stomatal conductivity of transgenic eggplant when treated with 200 mM NaCl (Yarra and Kirti 2019), further supporting of TaNHX2 gene role as an important regulatory factor in conferring salinity tolerance. Moreover, induction of tonoplast Na+/H+ antiporter activity and transcription levels has been demonstrated in enormous salt-tolerant plants under high salinity (Blumwald et al. 2000; Hamada et al. 2001; Shi and Zhu 2002; Yu et al. 2007; Tang et al. 2010; Silva et al. 2010; Chakraborty et al. 2012; Upadhyay et al. 2012; Pitann et al. 2013; Liu et al. 2013; Lu et al. 2014; Jaarsma and de Boer, 2018). These works clearly indicate a central role of Na+/H+ antiporter in Na+ sequestration, ion homeostasis, and salinity tolerance in a wide range of plants. Taken together, it is evident that salinity stress enhances Na+/H+ antiporter NHX1 expression and activity to play a crucial role not only in in Na+ accumulation in the vacuoles but also in K+ homoeostasis and thus resulting in salinity tolerance.
Evidence for NHX Expression Mediating Na+ Accumulation Under Salinity
A positive link has been frequently observed between Na+ accumulation and NHX expression in roots and shoots of salt-tolerant plants. For instance, salt-tolerant bread wheat cultivar, relative to the sensitive one, had an enhanced ability to sequester large quantities of Na+ into the vacuoles of root cells, which was consistent with the highest level of expression of NHX1 transcripts in plant roots under salinity exposure (Cuin et al. 2011). Also, increased salinity tolerance of SlSOS2-overexpressing tomato plants was associated with higher Na+ content in stems and leaves and with the induction and upregulation of vacuolar K+/H+ and Na+/H+ (LeNHX2 and LeNHX4) antiporters, responsible for Na+ loading into the xylem and Na+ and K+ compartmentalization (Huertas et al. 2012). Further evidence is provided by the finding that CiNHX1 plays a critical role in chicory tolerance to salinity stress, as a link between Na+ content and CiNHX1 expression in the leaves and roots has been shown during exposure to salinity, implying CiNHX1 functions as a transporter that moves Na+ into the vacuole to alleviate ionic toxicity to the cytoplasm (Liang et al. 2015). In accordance, a significant positive correlation was observed between Na+ accumulations and IlNHX expression in Iris lactea tissues under 200 mM NaCl, indicating IlNHX responsibility for Na+ accumulation in the vacuoles under salinity stress (Guo et al. 2020). This study revealed also that transgenic tobacco expressing IlNHX grew better and showed higher tolerance to 200 mM NaCl than wild type by accumulating more Na+ and K+, maintaining higher K+/Na+ ratios and chlorophyll content in tissues, showing higher V-ATPase activity and reducing lipid peroxidation in the presence of NaCl stress. It is obvious that higher activity of tonoplast pump energizes vacuolar NHX to sequester excess Na+ and K+ into the vacuole, which possibly contributes to osmotic adjustment. A recent study revealed also that salt-tolerant rice genotypes accumulated much higher Na+ in the roots than sensitive genotypes (Theerawitaya et al. 2021). Salt-tolerant rice genotypes had lower Na+ in the shoots relative to the roots. These observations were related to increased expression level of OsNHX3 in the roots to sequester Na+ into the vacuole as well as low translocation rate from root-to-shoot tissues by downregulation of various ion transporters, such as OsHKT1;5. It is clear from this work that salt-tolerant rice genotypes thus prevented Na+ toxicity to the cytoplasm of root cells as well as photosynthetically active leaves under high salinity. Similarly, although acclimation of pea plants to salinity takes place primary in the root tissues, control of xylem ion loading and efficient sequestration of high Na+ in mesophyll cells were also important components of this process (Pandolfi et al. 2012). In this research, shoot high Na+ was used as a cheap osmoticum resulting in water retention required for turgor maintenance and cell expansion. In a subsequent work by Pandolfi et al. (2016), Na+ exclusion from uptake has been proved to be of a much less importance compared with the efficient vacuolar Na+ sequestration in the shoot of acclimated maize plants, as a key trait for salinity tolerance. Acclimated maize plants also retain more K+ but less Na+ in roots and use accumulated Na+ similarly for osmotic balance in the shoot.
In accordance with the above evidence, a recent work by Wu et al. (2019) consistently reported that the PM Na+/H+ antiporter-mediated Na+ extrusion from the root of 45 barley accessions plays a minor role in the overall salinity tolerance, but a strong positive correlation was found between root vacuolar Na+ sequestration ability and salinity tolerance. In the same trend, the response of the root PM ATPase (energizes the PM Na+/H+ antiporter) was not correlated to salinity tolerance of wheat cultivars contrasting in their response to saline conditions, since the root PM proton pumps responded similarly (i.e., reduced activity of both ATPases) to NaCl stress in both wheat cultivars (Mansour et al. 1998). Hossain et al. (2017) also report that the regulatory mechanism of cytosolic K+/Na+ homeostasis is an important salinity tolerance determinant in salt-tolerant rice, because tolerant genotype maintains cytosolic ion homeostasis which increases K+/Na+ ratio by the induction of the expression of OsNHX1 and OsNHX2 genes, increasing the compartmentalization of cytosolic Na+ into the vacuole. A clear association between High Na+ and induced NHX1 expression is evident in this investigation as well as others presented previously. A study by Yuan et al. (2015) clearly confirms that ZxNHX is essential for controlling Na+ and K+ accumulation and salinity resistance in the halophyte Zygophyllum xanthoxylum; this is because ZxNHX regulates the gene expression involved in Na+ and K+ transport and spatial distribution under NaCl treatment (Table 2). Moreover, the role of NHX-type vacuolar antiporters as determinants of salinity tolerance in tomato was also confirmed by Galvez et al. (2012) who showed that salt-tolerant varieties displayed enhanced expression of LeNHX3 and LeNHX4 with a concomitant increase in Na+ in their tissues during salinity stress. It is interesting to mention that another research reports that short-term salinity treatment results in apoplastic Na+ accumulation, but not vacuolar accumulation in Populus euphratica plantlets, but the plantlets showed a higher accumulation of Na+ in the vacuole than in the cytosol after 9 weeks of 150 mM NaCl exposure (Ottow et al. 2005a, b). The results suggest that Na+ accumulation in the vacuole may be tissue specific, salt level and duration, and plant developmental state dependent. In this work, salinity stress enhances also Na+/H+ antiporter expression and activity, supporting the proposed Na+ accumulation in the vacuole. Similarly, more Na+ accumulation in leaves of salt-tolerant barley genotype (Shabala et al. 2010) and in root of salt-tolerant wheat varieties (Wu et al. 2015) compared with sensitive ones has been reported in response to salinity stress. In these barley-tolerant genotypes, high Na+ is supposedly accumulated in vacuoles and participated in osmotic adjustment. Accordingly, we present a further line of evidence supporting an association between NHXs expression and vacuolar Na+ accumulation and also NHXs contribution to salinity tolerance by favorably adjusting cellular K+ homeostasis under saline conditions.
Overexpression of NHX-Encoding Genes Enhanced Salinity Tolerance
Another line of evidence is the impact of NHXs overexpression on mitigating the salinity adverse effects and improving tolerance to saline conditions in various plant species. For example, AtNHX1 overexpression in salt-stressed transgenic tomato plants resulted in increased vacuolar Na+/H+ and K+/H+ exchange and mediated Na+ sequestration into the vacuole, which enhanced salinity tolerance and elevated fruit production at 200 mM NaCl (Zhang and Blumwald 2001). It is noteworthy to mention that fruits quality and yield of transgenic tomato in response to salinity were similar to control plants under NaCl treatment. Consistently, overexpression of AtNHX1 gene in tomato plants enhanced their tolerance to salinity stress by retaining high intracellular K+ level and increasing proline and sugar accumulation in the cytosol (Leidi et al. 2010). This work also showed that overexpression of the AtNHX1 in tomato induced K+ deficiency symptoms despite transgenic plants having greater K+ contents than controls. It is proposed that the intense sequestration of K+ in NHX1-overexpressing plants reduced cytosolic K+ activity, primed the induction of the high-affinity K+ uptake system, and elicited an array of metabolic and hormonal disorders related to K+ deprivation. In addition, transgenic tomato plants overexpressing a Na+/H+ exchanger gene (TNHXS1), singly or with V-PPase gene showed higher salinity tolerance than the wild-type plants by producing higher biomass and retaining more chlorophyll, catalase activity, earlier flowering, and more fruits under NaCl stress (Gouiaa and Khoudi 2015). Wu et al. (2016) also reported that soybean plants exposed to 300 mM NaCl showed enhanced salinity tolerance when AtNHX5 was overexpressed; the enhancement effect was through accumulating proline, transporting Na+ and K+ from the roots to the leaves, and lowering lipid peroxidation. Furthermore, overexpression of RtNHX1 enhanced seed germination, biomass accumulation, chlorophyll content, root elongation, more K+ and less Na+ in leaves, lower Na+/K+ ratios, higher antioxidant enzyme activities, proline content, RWC, and decreased lipid peroxidation in transgenic Arabidopsis plants under saline stress (Li et al. 2017, Table 2). It seems that ion homeostasis and enhancing osmotic and antioxidant regulatory capacity are main strategies regulated by RtNHX1 expression to render adaption to salinity stress in the work of Li et al. (2017). Supporting to the fact that AtNHX1 plays a role in K+ accumulation in the vacuole is the finding that overexpression of TaNHX2 gene confers salinity tolerance in transgenic alfalfa by increasing the retention capacity of intracellular K+, which leads the authors to suggest that the intracellular compartmentalization of K+ is critical for TaNHX2-induced salinity tolerance in transgenic alfalfa (Zhang et al. 2015a). Also, hydroponics and soil culture experiments carried out by Zeng et al. (2018) demonstrated that the expression of HtNHX1 or HtNHX2 improved rice tolerance to high salinity, while expression of HtNHX2, but not HtNHX1, improved rice growth and grain yield. The study also revealed HtNHX2 localization to intracellular compartments other than the vacuole, which causes more accumulation of Na+ and K+ in HtNHX1 plants. These findings suggest that different subcellular localizations of both NHX types appear to induce different mechanisms of salinity tolerance. In support, PgNHX genes displayed tissue-specific expressional patterns in pomegranate, with relatively low expression levels in roots and high expression levels in leaves under different concentrations of NaCl stress (Dong et al. 2021a). This expressional pattern might alleviate the harmful effects of Na+ via sequestrating the excessive Na+ into vacuoles of leaves and reducing the Na+ accumulation in roots and hence has an adaptive value in salinity resistance.
Further evidence is provided by a study carried out by Bulle et al. (2016) showing that introducing the wheat Na+/H+ antiporter gene (TaNHX2) into Capsicum annuum enhanced salinity tolerance of transgenic plants by elevating proline level, chlorophyll, antioxidant enzymes, RWC, and reducing levels of H2O2 and MDA. Consistently, transgenic switchgrass-overexpressing PvNHX1 showed higher shoot height, larger stem diameter, longer leaf length, and width, increased proline accumulation, and preserved cell membrane integrity under salinity stress, suggesting PvNHX1 is essential for normal plant growth and development and play an important role in the response to salinity stress by improving K+ accumulation (Huang et al. 2017). Qiao et al. (2011) also demonstrated that overexpression of AtNHX1 improved salinity tolerance in a transgenic poplar by accumulation of Na+, maintained chlorophyll content, and decreased lipid peroxidation under salinity. In this research, overproduction of a vacuolar Na+/H+ antiporter potentially increases Na+ sequestration, thereby avoiding Na+-specific toxicity and used for osmotic balance. A recent study similarly showed that overexpression of LeNHX4 improved yield, fruit quality, and salinity tolerance in tomato plants through accumulating higher contents of sugars, proline, proteins, Na+, and K+ (Maach et al. 2020). The results point out to Na+ sequestration and osmotic adjustment through higher accumulation of Na+, K+, proline, sugars, and proteins. Moreover, when SbNHX1 gene from Salicornia brachiate was introduced into Jatropha curcas, transgenic lines showed better salinity tolerance by vacuolar membrane-bound SbNHX1 involvement in Na+ compartmentation and ion homeostasis in transgenics and thus alleviating the adverse impacts of 200 mM NaCl (Jha et al. 2013). Based on the above observations, improved salinity tolerance and increased accumulation of Na+ or K+ were shown when NHX was expressed in a variety of plant species. The aforementioned findings also indicate the key role of the vacuolar Na+/H+ antiporter in Na+ detoxification, ion and osmotic homeostases, and hence tolerance to saline conditions.
Studies given in this section are further confirming the fact that engineering plants to overexpress NHX genes is an effective strategy for generating salinity-tolerant plants under saline stress. For instance, overexpression of NsNHX1 gene from the halophyte Nitraria sibirica into Arabidopsis enhanced salinity tolerance in transgenic plants, suggesting NsNHX1 important role in compartmentalization of Na+ into the vacuole (Wang et al. 2016a, b). Banjara et al. (2012) revealed also that expression of Arabidopsis Na+/H+ antiporter gene (AtNHX1) in peanut improved salinity tolerance of the transgenic plants which suffered less damage, produced more biomass, contained more chlorophyll, and maintained higher photosynthetic rates after salinity exposure. Additionally, NHX gene (AhNHX1) from Arachis hypogaea introduced into tobacco improved salinity tolerance of tobacco seedlings, with higher K+/H+ antiporter activity under salinity (Zhang et al. 2017b, Table 2); the transgenic tobacco seedlings possessed higher K+ accumulation in the roots, stems, and leaves, but not Na+ accumulation and thus leads to a higher K+/Na+ ratio after NaCl treatment. The study demonstrated also that V-ATPase and V-PPase activities were higher in transgenic seedlings under NaCl stress, confirming their essential role in energizing the NHX exchange process. These results clearly indicate that AhNHX1 catalyzed the K+/H+ antiporter and enhanced tobacco tolerance to salinity stress by increasing K+ uptake and transport. Similarly, Assaha et al. (2017) report that AtNHX1 and AtNHX2 function primarily as K+/H+ transporters to mediate vacuolar K+ accumulation and thus affecting transpiration rate via regulation of stomatal function. Another study by Rodriguez-Rosales et al. (2008) showed that transgenic tomato-overexpressing LeNHX2 mediates K+ accumulation and confers salinity resistance. Further, transgenic alfalfa plants co-expressing ZxNHX and ZxVP1-1 grew better with greater plant height, dry mass under, higher leaf RWC, greater photosynthesis capacity, more Na+, K+, and Ca2+ accumulation in leaves and roots compared with wild-type plants under NaCl stress in the greenhouse and field conditions (Bao et al. 2016). Moreover, overexpressing vacuolar Na+/H+ antiporter gene OsNHX1 in rice (Amin et al. 2016), TaNHX3 in tobacco (Lu et al. 2014), AtNHX1 in sweet potato (Fan et al. 2015), AtNHX1 in wheat (Xue et al. 2004), and OsNHX1 in rice (Chen et al. 2007) have been illustrated to enhance salinity tolerance in transgenic plants in response to saline conditions. These transgenic plants showed increased Na+/H+ exchange activity, high K+/Na+ ratio, activities of antioxidant enzymes, chlorophyll content, yield and different organic osmolytes, and lower lipid peroxidation under high salinity. It is most likely that high Na+ sequestered into the vacuoles and used as a cheap solute in osmotic adjustment for water uptake and turgor maintenance. In the same trend, Bassil et al. (2011) observed lower vacuole pH and K+ concentration in the nhx1 nhx2 mutant, further supporting the role of AtNHX1 and AtNHX2 in driving the uptake of K+ into the vacuole. In the above studies, it is important to note that high vacuolar K+ in addition to act as a cheap osmolyte, K+ may have an adaptive significance since vacuolar K+ ions are subsequently recycled between cytoplasm and vacuole (Jiang et al. 2010). Potassium is involved in numerous physiological functions, including plant water relations, transpiration, cell turgor maintenance, stomatal opening and closing, assimilate translocation, phloem translocation, enzyme activation, and leaf movements (Jordi Sardans and Peñuelas 2021).
Several investigations conducted on the model plant Arabidopsis also demonstrate that when genes encoding NHX proteins isolated from different plant species and introduced in Arabidopsis effectively alleviated salinity stress effects in the transgenic plants. A recent study indicated that transgenic Arabidopsis plants overexpressing Na+/H+ antiporter gene from date palm (PdNHX6) showed enhanced tolerance to salinity, which was associated with a higher chlorophyll accumulation, water content, and seed germination rates when compared with control plants (Al‑Harrasi et al. 2020). Interestingly, despite the significant increase of Na+, transgenic Arabidopsis maintained a balanced Na+/K+ ratio under saline conditions, which led the authors to conclude that PdNHX6 enhanced salinity tolerance through K+ and vacuolar pH homeostasis. Another research showed that overexpression of a vacuolar NHX1 gene from mung bean (VrNHX1) in Arabidopsis increased tolerance to salinity by increasing the K+/Na+ ratio, enhancing proline accumulation, and reducing MDA level (Mishra et al. 2014). In addition, Sun et al. (2019) recently reported that soybean Na+/H+ exchanger GmNHX1 responds and regulates plant tolerance to salinity imposition; transformed Arabidopsis-expressed GmNHX1 showed changes in the flow rate of K+ and Na+ in root cells by altering the expression of SKOR, SOS1, and AKT1 in order to regulate the accumulation of K+ and Na+ in roots and leaves as well as the maintenance of a high K+/Na+ ratio in roots under saline conditions. It is clear from this work that GmNHX1 regulated the above stress-related genes, in addition to controlling Na+ sequestration into the vacuole and hence improving tolerance to high salinity. It is interesting to mention that the tonoplast Na+/H+ exchanger in Arabidopsis is regulated by the SOS pathway because activated SOS2 protein added in vitro increased tonoplast Na+/H+-exchange activity in vesicles isolated from sos2 but did not have any effect on activity in vesicles isolated from wild type, sos1, or sos3 (Qiu et al. 2004). As such, SOS2 interacts with and activates the V-ATPase, VHA (Pardo and Rubio 2011), and consequently SOS2 may indirectly affect the activity of NHX exchangers through the regulation of V-ATPase activity (Fig. 3). In the same trend, PtCBL10, calcineurin B-like protein, has been revealed to play important role in poplar salinity tolerance through interacting with the salt tolerance component PtSOS2; this reaction is associated with the vacuolar membrane (Tang et al. 2014). Overexpression of either PtCBL10A or PtCBL10B conferred salinity tolerance on transgenic poplar plants by maintaining ion homeostasis in shoot tissues (differentially regulates tissue Na+ and K+ distribution) under salinity stress. The data underline the central role of SOS2 (as well as other effectors) as a hub to activate transport proteins that remove Na+ from the cytosol into the vacuole and to activate proteins that provide energy for these transport activities. Taken together, the results strongly suggested that the vacuolar NHXs plays a crucial role not only in in Na+ accumulation in the vacuoles but also in pH regulation, K+ homoeostasis, as well as regulation of other salt-responsive genes under salinity stress.
Co-expression of NHX Genes with Other Genes Produced Efficient and Strong Transgenes Under High Salinity
Transformation of more than one gene has been strongly efficient in enhancing tolerance to saline imposition in different plant species, although transgenes expressing single gene have proven, to some extent, to alleviate salinity harmful effects. One common gene that has been found to be implicated in this gene transformation is NHX1. For example, the TaNHX1expression, together with TVP1, a wheat H+-PPase gene in Arabidopsis, improved plant resistance to high NaCl stress by accumulating more Na+ and K+ in their leaf tissue, high K+/Na+ ratio, and more negative water potential compared with wild-type plants (Brini et al. 2007). These results indicate that the toxic effect of Na+ accumulation in the cytosol is most likely reduced by its sequestration into the vacuole. In addition to the PM Na+/H+ transporter SOS1, HKT-type protein, tonoplast Na+/H+ antiporter NHX1 has shown as a key Na+ transporter involved in salinity tolerance of halophytic grass Puccinellia tenuiflora since tonoplast Na+/H+ antiporter NHX1 expression was increased in the shoots and roots under high salinity (Zhang et al. 2017a). The study also revealed that under mild salinity, PtNHX1 in shoots compartmentalizes Na+ into vacuole slowly, which then enhances Na+ loading into the xylem of roots by PtSOS1 through feedback regulation and consequently, Na+ could be transported into shoots by transpiration stream for osmotic adjustment. Under severe salinity, Na+ was, however, rapidly and persistently sequestrated into vacuoles by PtNHX1, which in turn restricts long-distance Na+ transport from roots to shoots and alleviates Na+ toxicity in photosynthetic tissues. This work confirms that the function of NHX1 gene might be dependent on stress duration and degree as well as tissue specific. Additionally, Arabidopsis-overexpressing vacuolar Na+/H+ antiporter AtNHX1 or the PM Na+/H+ antiporter SOS1 improves salinity tolerance in transgenic plants only when NaCl concentrations are lower than 200 mM (Pehlivan et al. 2016). Interestingly, co-overexpressing AtNHX1 and AtSOS1 improved salinity tolerance in transgenic Arabidopsis up to 250 mM NaCl. The stimulatory effect was much higher in the transgenic plants overexpressing both genes than those overexpressing a single gene AtNHX1 or AtSOS1 (Pehlivan et al. 2016). These findings confirm the synergistically modulation of Na+ homeostasis as well as multigenic nature of salinity resistance. Similarly, when PutNHX1 and SeNHX1 from halophytes Puccinellia tenuiflora and Salicornia europaea expressed in Arabidopsis wild-type Col-0 and the nhx1 mutant, transgenic lines sequestered large quantity of Na+ into root cell vacuoles and also promoted high cytosolic and vacuolar K+ accumulation, while the nhx1 mutant pumps Na+ out of the cell (Liu et al. 2017). The results indicate that in addition to other regulatory mechanisms, Na+ sequestration in the vacuole and K+ retention in the cytosol and vacuole of root cells are crucial traits for plant tolerance to saline environment. A recent study carried out by Baghour et al. (2019) showed that joint overexpression of LeNHX2 and SlSOS2 improves growth and water status under NaCl stress, affects K+ and Na+ homeostasis, and enhances fruit yield of tomato plants. The study similarly revealed that plants overexpressing both LeNHX2 and SlSOS2 showed better salinity tolerance under saline conditions than plants overexpressing only one of these genes or wild-type plants.
Consistent with the previous evidence, Maach et al. (2020) revealed that when LeNHX2 and SlSOS2 co-overexpressed in tomato plants, transgenic tomato plants grew better, showing a higher biomass, higher yield and fruit quality, higher proline, glucose, and protein contents in leaves as well as pH and total soluble solid in fruits compared with wild-type plants under saline conditions. Also, co-expressing of ZxNHX and ZxVP1-1 genes from Zygophyllum xanthoxylum in Lotus corniculatus improved salinity tolerance of the transgenic lines by maintaining membrane integrity, higher photosynthetic rate, and higher Na+, K+, and Ca2+ accumulation in the leaves and roots causing lower leaf solute potential and thus more water retention in response to 200 mM NaCl stress (Bao et al. 2013). The work similarly demonstrated that co-expression of both genes confers greater salinity tolerance than expression of a single gene (ZxVP1-1). In this context, the pyramiding between salt-responsive protein 3–1 (SRP3-1) and V-ATPase subunit c1 (VHAc1) of Spartina alterniflora in rice increased the production of grain per plant, a parameter highly appreciated by agriculture (Biradar et al. 2018). Recently, Barros et al. (2021) also present evidence that the transformation must include as many genes as possible so that the response can be more representative and strongly enhanced in response to salinity stress. Furthermore, the improved salt tolerance of lettuce cultivars inoculated with AM fungi was closely related to increased PIP2 abundance, as well as to the upregulation of LsaNHX gene expression, which concomitantly improved plant nutrition and maintenance of K+/Na+ homeostasis (Santander et al. 2020). In a similar way, AM symbiosis in rice plants improved salinity tolerance by upregulating the OsNHX3 isoform, influencing the Na+ compartmentalization into the vacuole, and increasing the efflux of Na+ from cytosol to apoplastic spaces via a high expression of OsSOS1(Porcel et al. 2016). The research also showed a decrease of Na+ root-to-shoot distribution and an increase of Na+ accumulation in rice roots, suggesting more than one gene cooperatively stimulate adaptive responses to salinity stress. A study carried out by Yuan et al. (2015) similarly demonstrated a vital role of ZxNHX in ion homeostasis and salinity tolerance of Zygophyllum xanthoxylum through regulating the expression of other salt-inducible genes (ZxSOS1, ZxHKT1;1, ZxAKT1, ZxSKOR) involved in Na+ and K+ transport under saline conditions. The above studies clearly indicate that strong salinity tolerance is achieved by synergistic impact of more than one gene, which explains why the salinity tolerance trait has multi-component nature. The activity of Na+/H+ antiporter was coordinated with that of ATPase of the tonoplast in leaves of Suaeda salsa under NaCl stress (Qiu et al. 2007), pointing out to the fact that the activity of the tonoplast proton pump is required to energize the tonoplast for Na+ compartmentation into the vacuole. In this study, upregulation of the vacuolar membrane Na+/H+ antiport activity was attributed to the increase in both transcription and translation. Based on the above findings, the efficiency of transgene pyramiding of more than one gene is evident for developing better genotypes with improved salinity tolerance and productivity in saline soils.
NHX-negative Correlation With Salinity Tolerance
Despite the established involvement of tonoplast Na+/H+ antiporter in mediating Na+ sequestration into the vacuole and K+ homeostasis, inconsistencies exist in some of the published information. We assume this may be due to differences in cultivars, experimental conditions, salt level used and duration, plant age, and studied tissue. For example, there was no strong correlation between GhNHX1 expression levels and salinity tolerance in cotton and thus Na+ sequestration into vacuoles by GhNHX1 might play an important, but non-essential role in the underlying genetic variability of salinity tolerance in cotton (Wang et al. 2017a). Also, no difference in expression level of Na+/H+ antiporter gene NHX2 between acclimated and non-acclimated wheat plants under high salinity (Janda et al. 2016), suggesting that this gene does not play a decisive role in salinity acclimation processes. In accordance, Adem et al. (2014) showed that NHXs cannot be used to indicate salinity tolerance in barley in response to saline conditions, as NHX1 and NHX2 transcript levels were higher in leaves and roots of salt-sensitive barley cultivars compared with tolerant one. Similarly, in a subsequent study by Adem et al. (2015), expressing AtNHX1 in barley does not improve plant performance under saline conditions. In this research, when Arabidopsis AtNHX1 gene was introduced into barley plants grown under saline conditions, lack of phenotype was shown which suggests absence of beneficial impact of expressing of AtNHX1 gene in barley (Adem et al. 2015). The lack of phenotype was thought to be possibly explained by (a) insufficient proton gradient required for vacuolar Na+/H+ antiporter performance due to low activity of both vacuolar proton pumps, (b) transgenic plants inability to prevent Na+ leak back from the vacuole via SV and FV channels, (c) insufficient ATP pool required for vacuolar proton pumps activity, and (d) possible misfolding, incorrect targeting, and inactivity of the AtNHX1 proteins. Moreover, NHX higher activity or overexpression of NHX genes in response to salinity stress does not always ensure improved salinity tolerance unless an efficient control of vacuolar leak channels (i.e., SV and FV channels) is warranted (Adem et al. 2015; Shabala et al. 2020). In spite of these few discrepancies, the crucial role of efficient vacuolar Na+ sequestration by vacuolar Na+/H+ antiporter is evident to account for the differential salinity tolerance in numerous plant species under saline conditions.
Vacuolar ion Channels and Tolerance to Saline Conditions
Types, Structure, Functions, and Overexpression of Vacuolar Ion Channels
Although numerous ion channels have been characterized in the tonoplast, three major types of cation channels are usually identified in plants, including mainly two-pore K+ channels (TPK1), slowly activated vacuolar (SV) and the fast-activated vacuolar (FV) channels, chloride channel proteins (CLC), and much less-studied aluminum-activated malate transporter (ALMT) proteins (Fig. 3, Shabala et al. 2020). These tonoplast ion channels have been revealed to play central role in enhancing plant adaptation to salinity stress. In addition, many works reported that overexpression of these different vacuolar ion channels largely contribute to promoting plant salinity tolerance in response to saline soil (Zhang et al. 2015a; Nguyen et al. 2016; Baetz et al. 2016; Hu et al. 2017; Dong et al. 2021b).
Tonoplast Two-pore K+ Channels (TPK1)
One of these tonoplast ion channels is the K+-selective vacuolar (VK) channel, a voltage-independent Ca2+-activated K+-selective channel encoded by the tonoplast two-pore K+ channels (TPK1) TPK1 gene (Voelker et al. 2010; Saibi and Brini 2021). The Arabidopsis genome contains 5 TPK isoforms, four isoforms are highly homologous (TPK1, 2, 3 and 5) and localize to the tonoplast, whereas TPK4 is expressed at the PM (Isayenkov et al. 2010). TPK1 currents are also regulated by calcium-dependent protein kinases (CDPKs) and 14-3-3 protein binding. For example, 14-3-3 proteins regulate K+ channel activity at the vacuolar membrane of barley mesophyll cells (Voelker et al. 2010, Fig. 3). This regulation of TPK1 allows maintaining cytosolic K+ homeostasis, particularly in response to salinity stress. Assaha et al. (2017) reported that the TPK1 channels are indeed important in replenishing lost cytosolic K+ from vacuolar pools under salinity stress. However, this phenomenon depletes vacuolar K+ pools and consequently decreases cell turgor due to absence of osmotic balance (Barragán et al. 2012). Therefore, the retention of cellular K+ should be a salinity tolerance determinant. Actually, overexpression of the Arabidopsis AtNHX1 in tomato improved salinity tolerance by mediating vacuolar K+ and not Na+ sequestration and thus retains intracellular K+, with a concurrent accumulation of proline and sugars in the cytosol (Leidi et al. 2010; Yaish 2015). Consistently, transgenic alfalfa overexpressing TaNHX2 decreased K+ efflux and thus retains more intracellular K+ under saline conditions (Zhang et al. 2015a), whereas nhx1nhx2 double mutants of Arabidopsis lacked the ability to create vacuolar K+ pools resulting in turgor loss and sensitivity to saline stress. In this context, Voelker et al. (2010) speculated that plant TPKs are targets of external and internal stimuli to fine-tune the electrical properties of the tonoplast for specialized transport tasks, e.g., K+ homeostasis under salinity stress.
Slowly Activated Vacuolar (SV) and the Fast-activated Vacuolar (FV) Channels
Another major group of vacuolar channels are not selective, with equal or similar permeability for Na+ and K+ (Shabala et al. 2020). These are the slowly activated vacuolar (SV) and the fast-activated vacuolar (FV) channels (Assaha et al. 2017; Shabala et al. 2020). SV channels are the most abundant and firstly discovered voltage-dependent ion channels in plant vacuoles and are permeable to both monovalent and divalent cations (Koselski et al. 2019). However, whether SV channels are Ca2+ channel permeates Ca2+ or Ca2+ is only an effector of SV channel gating is a matter of controversy (Hedrich et al. 2018). Contrary, FV channels are permeable for monovalent cations only (Shabala et al. 2020). SV channels strongly accommodate cation fluxes from the cytosol to the vacuole and have roles in cell nutrition and signaling (Pottosin and Dobrovinskaya 2014). SV channels are encoded by TPC1 (two-pore channel 1) and have been shown to localize to the tonoplast in Arabidopsis (Saibi and Brini 2021, Table 3). They are activated by cytosolic Ca2+ concentration exceeding 10 µM but inhibited by luminal Ca2+, dominate vacuolar Ca2+ conductance, and its relative expression determines the vacuolar Ca2+ storage capacity (Demidchik et al. 2018; Koselski et al. 2019; Dreyer et al. 2021). TPC1 channels mediate Ca2+ release from the vacuole for signaling needs and are also essential components in long-distance Ca2+ signaling as well (Demidchik et al. 2018). Current thinking is therefore that TPC1 is part of a Ca2+/ROS relay that propagates stress signals (Evans et al. 2016). A recent study shows that TPC1 is activated only in the presence of K+, but Na+ increase would inhibit the activity of SV channels (Jaślan et al. 2019). In addition, it is important to mention that besides to Ca2+, SV channels are regulated by numerous factors playing roles in cell nutrition and signaling, such as Mg2+, Zn2+, H+, polyamines, dithiothreitol, glutathione, 14-3-3 proteins, heavy metals, phosphorylation/dephosphorylation, calmodulin, and H2O2 (Hedrich et al. 2018; Koselski et al. 2019). Accordingly, it can be speculated that these factors might indirectly influence plant response and tolerance to salinity via their regulatory role on the activity of SV channels. Further, FV channels are partly suppressed by cytosolic Ca2+, Mg2+, and polyamines, implying that it is their regulation rather than the number of copies that is essential for salinity tolerance (Shabala et al. 2020). However, these ion channels still need further research to elucidate and document their involvement in plant adaptation to saline stress.
The following investigations support the role that TPC1 channels may play under saline stress. First, the study of Koselski et al. (2019) concluded that the SV channels participate in salinity stress adaptation of the liverwort Conocephalum conicum because they are permeable to Na+ and hence can sequester Na+ inside the vacuole. The study also showed the ability of these channels to release excess Na+ from the vacuole into the cytoplasm, which is subsequently expelled from the cytoplasm to the extracellular space by the PM Na+ transport mechanisms. Extrusion of stored Na+ in the vacuole is possibly to avoid cytoplasmic dehydration when the cell is unable to produce sufficient organic osmolytes and/or K+ in the cytoplasm to balance reduced vacuolar osmotic potential under salinity. Second, when Bonales-Alatorre et al. (2013a) studied FV and SV channel activity in young and old leaves of quinoa under salinity stress, they showed that closed SV and reduced FV channel activity in old leaves enhanced vacuolar Na+ retention, which corresponds with enhanced salinity tolerance. In another study by Bonales-Alatorre et al. (2013b), they demonstrated that the salt-tolerant quinoa genotype displaying low activity of the tonoplast FV and SV channels compared with high activity in the sensitive genotypes. It seems that difference in salinity tolerance between the two quinoa genotypes may be attributed to the ability of the tolerant genotype to prevent Na+ leakiness from the vacuole to the cytosol by reducing the activity of the tonoplast FV and SV channels in response to saline stress. In support, Assaha et al. (2017) indicated that equally important is the role of FV and SV channels that mediate vacuolar Na+ leakage to the cytosol, deemed a salt-sensitive trait. Therefore, SV channels may be involved in maintaining turgor and tonoplast potential by preventing Na+ leakage from the vacuole (Isayenkov et al. 2010). Consequently, it is obvious that although vacuolar sequestration of Na+ is very important strategy in the regulation of cytosolic Na+ accumulation, retention of the sequestered Na+ is equally proposed as a key stress tolerance mechanism because Na+ leakage back to the cytoplasm via the FV and SV channels has been associated with salinity sensitivity (Bonales-Alatorre et al. 2013a, b; Pottosin and Dobrovinskaya 2014; Wu et al. 2019). Furthermore, TPC1 channels contribute substantially to cellular K+ homeostasis, although plants lacking TPC1 function are not impaired in growth and development (Saibi and Brini 2021). This may indicate that the TPC1 channel is closed most of the time and opens upon specific inputs or under stress. One important issue raised here is that higher vacuolar channels’ activity requires a higher rate of the NHX transport to oppose it, which implies a higher ATP usage for futile cycling, whereas a plant ability to assimilate CO2 is severely limited under saline conditions (Shabala et al. 2020). Consequently, high-yield penalties will result, which indicates that overexpression of NHX genes does not necessarily mean improved salinity tolerance unless an efficient control of vacuolar leak channels is warranted (Adem et al. 2015; Shabala et al. 2020). Despite the above arguments, Shabala et al. (2020) proposed physiological significance for plants to have many leak channels at the tonoplast; this may include (a) SV and FV channels may be essential for turgor control and K+ homeostasis under non-saline conditions, (b) SV channel is the only established vacuolar Ca2+-permeable channel, and hence it determines the vacuolar Ca2+ storage capacity and intracellular Ca2+ availability and plays a key role in long-distance Ca2+ signaling, and (c) TPC1 channel may have a role as oxygen sensor that may be critical for plant adaptation to hypoxia and flooding. Taken together, the physiological roles of SV and FV channels, however, cannot be considered in isolation and have to be taken in conjunction with energy-consuming futile Na+ cycle. Future work should therefore focus on enabling their blockage in stressed plants, which will lead to enhanced retention of sequestered Na+ in the vacuole and hence enhanced salinity tolerance.
Vacuolar Chloride Channels (CLC)
Another crucial vacuolar ion channel that has been reported to develop plant salinity tolerance under saline conditions is chloride channel proteins (CLC). This is because Cl− is a critical component in salinity-induced ionic toxicity that causes plant salinity injury. Plants reduce the Cl− effects via active Cl− efflux or vacuolar Cl− partitioning to re-establish intracellular Cl− homeostasis (Tealle and Tyerman 2010; Wong et al. 2013; Wei et al. 2016). Munns and Tester (2008) even claimed that Cl− toxicity is more important than Na+ toxicity in some woody species, e.g., citrus. Similarly, Sun et al. (2009) noticed that the inability to restrict Cl− uptake contributes to the NaCl-induced salinity damage in salt-sensitive poplar species, which leads them to conclude that the differential response exhibited by plants to salinity stress is related to their ability to restrict Cl− transport to the aerial part. However, Cl− has been reported to be not only a cheap osmoticum but also a beneficial plant macronutrient (Wege et al. 2017; Maron 2019). Evidence indicates that anion channels and transporters are present at the tonoplast and are in charge of anion fluxes into the vacuoles. One of these anion channels are chloride channel proteins (CLC) characterized in Arabidopsis (AtCLCs: AtCLCa, AtCLCb, AtCLCc, AtCLCg) as well as other species to be localized in various membranes, including the vacuolar membrane (Wang et al. 2015; Wu and Li 2019; Fig. 3). The authors suggested these channels to be involved in the regulation of anion homeostasis (e.g., NO3−, Cl−) in plants under salinity. It is also assumed that these CLC proteins could be involved in processes that require Cl− transport, such as stomatal movement, pH adjustment, turgor pressure adjustment, hormone signal recognition, and transduction as well as abiotic and biotic stress tolerance (Tealle and Tyerman 2010; Wei et al. 2016). In response to salinity imposition, it is therefore expected that the treated plants utilize the anion transporters such as CLCs to adjust and reduce Cl− accumulation in the cell cytoplasm. In support to this assumption is the finding that the protein encoded by salt-inducible GmCLC1 gene localized on tonoplast has been shown to transport and sequester Cl− into the vacuoles of soybean cells allowing better tolerance to salinity in the transgenic BY2 cells (Li et al. 2006). Interestingly, other studies revealed also that the genetic differences in the control of Cl− transport from roots to shoots or the ability to maintain a low shoot Cl− level is the key determinant of salinity tolerance in different plant species (Zhang et al. 2011; Henderson et al. 2014). Diédhiou and Golldack (2006) reported that the transcription of the chloride channel gene OsCLC1 is repressed in the salt-sensitive rice line which accumulates greater Cl−, whereas the transcription is induced in the salt-tolerant rice line which excludes Cl− after NaCl treatment (Table 3). Also, overexpression of the tonoplast-located AtCLCc results in higher Cl− accumulation and increased overall salinity tolerance in the transgenic line relative to wild-type Arabidopsis (Nguyen et al. 2016; Hu et al. 2017). In support, atclcg-knock-out mutants showed a decrease in biomass and accumulate Cl− in shoots in the presence of NaCl, suggesting a physiological function of AtCLCg in the Cl− homeostasis during NaCl stress. The previous work also indicated that an atclcc/atclccg double mutants was not more sensitive to NaCl stress than the single mutant, which demonstrates that AtCLCc and AtCLCg formed part of a regulatory network controlling Cl− sensitivity and were both important for Cl− tolerance but not redundant. Consistently, Jossier et al. (2010) revealed that the atclcc-knock-out mutant was found to be more sensitive to salinity stress than the wild type and hence it was suggested that AtCLCc contributes to the detoxification of the cytosol by sequestrating Cl− into the vacuole. AtCLCc is mainly expressed in the tonoplast of the stomatal guard cells displays transmembrane Cl− transporting activity, aiding in the regulation of stomatal movements and enhancing salinity tolerance (Jossier et al. 2010). Furthermore, GmCLC1, soybean Cl−/H+ antiporter, enhanced salinity tolerance in transgenic Arabidopsis thaliana by compartmentalizing Cl− into vacuole and thus reduce the Cl− accumulation in shoots and release the negative impact of salinity stress on plant growth (Wei et al. 2016). Wong et al. (2013) further found that the transmembrane Cl− transport activity of the GmCLC1 protein depends on the cytoplasmic pH value, suggesting that it is most likely a kind of Cl−/H+ antiporter that participates in the maintenance of intracellular Cl− homeostasis and regulates salinity tolerance. In this research, overexpression of GmCLC1 in the hairy roots of soybean sequestered more Cl− in their roots and transferred less Cl− to their shoots, leading to lower relative electrolyte leakage values in the roots and leaves, the data suggesting a protective function of GmCLC1 under high salinity. A recent study carried out by Zhang et al. (2020b) revealed that maize was not able to re-translocate significant amounts of Cl− from shoot back to root and stored Cl− in sheaths of the old leaves and in roots under Cl− salinity. The authors assumed that vacuolar sequestration of Cl− in the roots might be a strategy to keep concentrations low in young growing shoot tissues and in leaf blades where photosynthesis is running. In this case, activity and/or expression of tonoplast CLCs are supposedly to be elevated under the stress condition to avoid Cl− cytoplasmic toxicity. Although little is known about the regulation of Cl− transport systems in plants under saline stress, it is clear that tonoplast CLCs are involved in salinity tolerance via regulating Cl− homeostasis.
Further observations support the implication of CLCs in plant response and tolerance to saline environments are provided here. For instance, NaCl-reduced expression of BvCLC-c and BvCLC-b in sugar beet was restored by exogenous boron, which elevates both genes expression levels (Dong et al. 2021b). It seems that tonoplast CLCs are possible reasons for boron-mediated reduction of cytosolic Cl− content through Cl− accumulation into vacuoles under NaCl stress, which improves the salinity resistance. Nakamura et al. (2006) showed also that OsCLC1, located at the tonoplast, was overexpressed in response to NaCl treatment in rice; the result is indicative of important role in dealing with excess Cl−. GsCLC-c2 overexpression in roots of wild soybean contributed to Cl− and NO3− homeostasis and therefore conferred salinity tolerance through increasing the accumulation of Cl− in the roots thereby reducing their transport to the shoots (Wei et al. 2019). It is worthy to note that GsCLC-c2 encodes protein that is more efficient as a chloride channel with higher permeability to Cl− than GmCLC1 of the cultivated soybean studied by Wei et al. (2016). In addition, Wu and Li (2019) argued that vacuolar Cl− sequestration could be possible component of plant salinity tolerance based on their experiments using positive tonoplast potentials to move Cl− from the cytosol to the vacuole through channels or transporters even if vacuolar Cl− is higher than that of the cytosol. These authors as well as others reported that electrical difference across the tonoplast varies from − 31 to + 50 mV; when a more negative tonoplast potential exists in salinity grown plants, this allows more efficient active transport of Cl− from the cytosol into the vacuole with the cost of ATP. In this case, Cl− transporters might play a role in Cl− storage in the vacuole. On the other hand, if positive tonoplast potentials predominate, the expense on ATP for active Cl− transport by Cl− transporters can be significantly reduced and thus can allow plants to have a longer time period of vacuolar Cl− sequestration under salt stress. Based on this finding, CLCs are cost effective and can alleviate salinity stress in plants through the sequestration of excess Cl− into the vacuoles of root cells and thus preventing Cl− from entering the shoots where it could result in cellular damages. In the same trend, works demonstrated that vacuolar Cl− channels accumulate high Cl− content in the vacuole after salinity treatment (Chen et al. 2002; Gu et al. 2004; Silva et al. 2010), which apparently contributes to osmotic balance to maintain water absorption and turgor under saline soils. It is important to mention that high vacuolar Cl− can induce the activity of V-ATPase and sustain the pH gradients and electrical excitability between the cytoplasm and vacuole, which contributes to cell membrane permeability and the elongation growth of vacuoles (Churchill and Sze 1984). Differential salinity tolerance between two wild edible greens was also due to accumulation of more Cl−, Na+, K+, and proline in the leaves of salinity-tolerant Reichardia picroides as compared with the sensitive Taraxacum officinale when treated with NaCl (Alexopoulos et al. 2021). It is most probably that these ions are used as a cheap osmolytes for osmotic adjustment to maintain water absorption and consequently turgor in response to NaCl stress. Also, accumulated proline under saline conditions has been reported to have antioxidant and other protective functions (Mansour and Ali 2017). In the study of Alexopoulos et al. (2021), it is implicitly suggested that tonoplast NHX and Cl− channels have been most likely activated to sequester both Na+ and Cl− into the vacuole. The previous findings inferred that CLC channels play vital roles in enabling plants to adapt to Cl− effects of salinity stress. Future studies should be, however, worked on dissecting the role of vacuolar channels in plant salinity tolerance.
Aluminum-activated Malate Transporters (ALMT)
Another tonoplast transporter that possibly contributes to Cl− accumulation in the vacuole in response to saline conditions is aluminum-activated malate transporter protein family (ALMT, Fig. 3). ALMT is unique to plants and able to mediate anion fluxes across cellular membranes including vacuolar sequestration of Cl− and hence salinity tolerance in plants (Wu and Li 2019). Angeli et al. (2013) have been demonstrated that AtALMT9, (a malate-activated Cl− channel located in the tonoplast), is involved in vacuolar Cl− sequestration and is also required for stomatal opening. Another study showed also that AtALMT9 is transcriptionally upregulated under salinity stress, whereas almt9-knockout mutants have reduced shoot accumulation of Cl− (Table 3, Baetz et al. 2016). Altogether, these limited results may suggest ALMT participation in vacuolar Cl− sequestration, which might be another component for overall plant salinity tolerance. However, large-scale experiments are still needed to validate this supposition.
Vacuolar Aquaporins (AQPs) and Tolerance to Saline Conditions
Types and Functions of Vacuolar Aquaporins
Aquaporins or water channels (AQPs) are a subfamily of major intrinsic proteins that regulate cellular water transport (Yan et al. 2020). Plant AQPs are designated as the PM intrinsic proteins (PIPs) or tonoplast intrinsic proteins (TIPs, Fig. 3). In Arabidopsis, there are five subgroups for TIP subfamily (TIP1, TIP2, TIP3, TIP4, and TIP5), based on their subcellular localization and sequence homology (Li et al. 2014b). In plants, AQPs are present in almost all organs, including the roots, leaves, stems, flowers, fruits, seeds, dry seeds, pollen, anther, and specific cells, such as guard cells (Kurowska 2021b). AQPs have proven therefore to support hydraulic regulation and nutrient transport, stomatal conductance, mesophyll conductance, transpiration and photosynthesis, growth and plant development, influence ionic homeostasis, and response to abiotic stresses (Kaldenhoff and Fischer 2006; Bezerra-Neto et al. 2019; Singh et al. 2020; Coelho et al. 2021). In particular, TIPs are considered as important elements of the mechanism that controls cell water homeostasis through the fast non-limiting water exchanges between the vacuole and cytoplasm (Martinez-Ballesta and Carvajal 2014; Singh et al. 2020). In addition to water transport, TIPs are capable of transporting some small neutral molecules that have great physiological significance such as glycerol, urea, ammonia, CO2, H2O2, formamide, and micronutrients (silicon and boron) across the cell and intercellular compartments (Rhee et al. 2017; Kurowska 2021a, b; Quiroga et al. 2020; Coelho et al. 2021). Transport of small solutes and gas may link TIPs to important metabolic pathways, like the urea cycle or amino acid synthesis. However, further direct evidence is still required to prove the subcellular partitioning of some of these small molecules mediated by the TIP family members. Besides being small solute and ion transporters, TIPs have also been found to be involved in salinity tolerance as they contribute to the accumulation of ions in vacuoles in response to salinity stress (Afzal et al. 2016). Abiotic stresses cause an imbalance in the water status in cells, tissues, and whole plants. Regulating the flow of water through the membranes is one of the main mechanisms via which cells can maintain their homeostasis under stress conditions and certainly AQPs are involved in this process to modulate the tissue hydraulic conductivity (Quiroga et al. 2020; Kurowska 2021b). As abiotic stresses cause a changing water status in plant cells resulting in cellular dehydration, aquaporins are hence thought to be responsive to the stress signaling pathways (Kurowska 2021a, b). Based on the above studies, TIPs have important diverse functions either under normal or stress conditions.
Response of TIP Genes to Salinity Stress
Reports have implied that TIP genes are associated with plant tolerance to abiotic stresses, such as drought and high salinity (Quiroga et al. 2020; Yan et al. 2020). For example, in Arabidopsis a significant reduction in hydraulic conductivity was coupled with a 60–75% decrease in PIP and TIP aquaporin transcripts abundance after exposure to salinity stress (Afzal et al. 2016), suggesting a relevant role in salinity tolerance. Similarly, arbuscular mycorrhizae-induced maize drought tolerance involved upregulation of tonoplast aquaporins, which have been shown to transport ammonium and/or urea under well watered and drought stress conditions (Quiroga et al. 2020). The investigation suggests a possible role of maize TIPs in drought tolerance. Under high salinity, upregulation of the gene expression of TIPs (in particular CpTIP2.4, CpTIP1.4, CpTIP1.3) was promoted to adjust plant water status and maintain water uptake by the root system in Calotropis procera (Coelho et al. 2021, Table 3). It is interesting to mention that salinity tolerance of C. procera begins with immediate regulation of aquaporin activity (in particular tonoplast aquaporins) in the root system. This possibly indicates TIPs involvement in salinity resistance by adjusting cell water flow (vacuole–cytosol–apoplast) and increasing water uptake by the root system. The authors proposed that gene expression of aquaporins supports the high performance of C. procera root system in response to salinity stress, which can act locally to protect cellular processes and maintain the activity of the root system through osmotic adjustment (accumulation of organic solutes), detoxification of ROS (accumulation of nonenzymatic antioxidants), and storage of cytosolic Na+ (high accumulation of Na+ in roots). Also, when Festuca arundinacea was exposed to 250 mM NaCl over 21 days, the abundance of the FaTIP1;1 transcript was increased in two contrasting salinity tolerance genotypes relative to the controls; this increase was observed on day six of the salinity stress in the tolerant genotypes, but on day 11 in the sensitive one (Table 3, Pawlowicz et al. 2017), pointing out to FaTIP1;1 implication in salinity stress response and tolerance. Consistently, high levels of aquaporin transcripts were found in salt-tolerant Malus hupehensis leaves which is beneficial for maintaining a better water status in leaves that is helpful for salinity tolerance (Liu et al. 2013). In the study of Senadheera et al. (2009), OsTIP1;1, a tonoplast-expressed aquaporin, was largely upregulated in salt-tolerant rice roots in response to salinity, suggesting that OsTIP1;1 contributes to osmotic homeostasis and salinity tolerance in rice. In addition, total tonoplast protein has been estimated to be composed of 15% V-ATPase, 10% V-PPase, and 40% TIP protein on the basis of protein amount for the tonoplast of mung bean hypocotyls (Maeshima 2001 and references therein). Similarly, among proteins identified in the tonoplast of the mangrove Avicennia officinalis, AQPs were predominant (Krishnamurthy et al. 2014). This research also showed involvement of tonoplast AQPs in ion homeostasis and turgor regulation. Due to a high abundance and a high specific activity of TIPs, compared with that of PIPs, water permeability of the tonoplast is markedly higher than that for the PM (Maeshima 2001), possibly to mediate cell water flow between vacuole, cytosol, and apoplast. Similar response has been illustrated in the work of Coelho et al. (2021). Furthermore, aquaporins have been recorded to be upregulated in Arabidopsis roots in response to salinity stress (Maathuis et al. 2003), indicative of their involvement in osmoregulation and non-limiting water flow through the tonoplast. In support to the TIPs involvement in plant salinity tolerance, the expression levels of tonoplast transport systems (including AQPs) are extremely low and not salinity inducible in salt-sensitive plants (Senadheera et al. 2009; Liu et al. 2013; Pawlowicz et al. 2017). It is clear that salinity-induced modulation of TIP transcript levels, proteins, and activity greatly influence several physiological processes that underpin salinity tolerance in salt-tolerant plants.
Regulation and Overexpression of TIP Genes Under Salinity Stress
As the activity of AQPs could be changed by the downregulation of the gene expression and AQPs gating regulation, Kurowska (2021b) reported that gene expression alterations and gene regulatory differences in response to environmental stress can be important components of the adaptation to stress conditions. For example, TIPs are under regulation of CsGPA1 (G-protein-alpha-1) as indicted by Yan et al. (2020) who showed that CsGPA1 interacts with CsTIP1.1 and resulted in opposite patterns of expression of CsTIP1.1 in leaves (increased) and roots (decreased), leading to declined water content of cucumber which reduces the availability of osmotic protectants and stimulates the production of deleterious ROS, ultimately conferring poor tolerance to salinity stress (Yan et al., 2020). The results suggest that CsGPA1 may act as a suppressor of CsTIP1.1 and confirm the role of GPA1 in the regulation of plant signaling during salinity stress.
The important role of TIPs in plant adaptation to saline stress comes from the findings that overexpression of TIPs has been associated with increased salinity tolerance in several plant species (Table 3; Kurowska 2021a; Coelho et al. 2021). For example, overexpression of the Panax ginseng aquaporin, PgTIP1, in Arabidopsis showed increased seed germination, plant growth, a higher accumulation of biomass, high Na+ content, and enhanced tolerance to salinity relative wild type (Peng et al. 2007). The authors concluded that PgTIP1;1 plays an important role in the growth and development of plant cells and suggested that its regulation to the water movement across the tonoplast has a great impact on plant vigor under favorable growth conditions and also in the response to salinity stress (Peng et al. 2007). Additionally, the tomato (Solanum lycopersicum) aquaporin gene SlTIP2;2 has been shown to positively regulate salinity response and tolerance by regulating cell water permeability and hence improving the vacuole osmotic buffering capacity of the cytoplasm, limiting the reduction in transpiration (Sade et al. 2009). This work also revealed that overexpression of SlTIP2;2 improves growth and yield production even under relatively severe stress conditions. The authors further suggest that the benefit of maintaining a certain amount of transpiration during stress, as opposed to a complete shutdown of transpiration, ensures not only continuous CO2 uptake but also a continued supply of nutrients and a reduction in leaf temperature, promoting plant growth. Similarly, transgenic Arabidopsis thaliana lines overexpressing JcPIP2;7 and JcTIP1;3 show improved germination, increased root length, seed viability, and yield under salt stress (Khan et al. 2015), which make these aquaporins important candidates for genetic manipulation of plants for growth in saline soils. Khan et al. (2015) also suggested that JcTIP1;3 probably functions more in maintaining cell turgidity and might interact more intricately with the cellular developmental and stress signaling machinery as has been reported for other TIPS. Also, overexpression of the CsTIP2-1 in tobacco increased the transgenic plant biomass more than wild type and control under salinity stress, which was correlated with the expansion of mesophyll cells (Martins et al. 2017). The previous works suggest that the TIPs might be involved in cell elongation. Moreover, some TIP genes (OsTIP1;1, OsTIP1;2) expressions were increased compared with the controls under salinity stress; this upregulation of some of OsTIPs genes might be connected with water transport from the vacuole to the cytoplasm to perform an osmotic adjustment (Li et al. 2008, Table 3). Based on the above observations, AQPs could play a role in the mechanism by which plants cope with saline stress because change in the AQP gene expression profiles regulates different metabolic and physiological aspects conferring tolerance to saline soil in many plant species. Also, TIPs might be a potential target in biotechnology and agriculture.
Differences in the experimental design, the nature, intensity and duration of the stress, and the developmental stage of the plants must be considered when studying plants overexpressing aquaporins under salinity stress. For instance, Arabidopsis plants overexpressing SlTIP2.2 show increased activity of antioxidant enzymes, high K+/Na+ ratio, enhanced osmotic regulation, and improved tolerance to salinity stress (Xin et al. 2014). The work also revealed a role for SlTIP2;2 in Na+ and/or K+ homeostasis and the tissue specific and NaCl dose-dependent regulation of SlTIP2;2 expression. It seems that SlTIP2;2 expression may have impacts on the expressions of antioxidant enzymes as well as effects on Na+ and K+ fluxes in addition to the regulation of membrane water permeability. Also, worthy of note is that the expressions of SlTIP2;2 in the roots of the wild-type tomato and transgenic Arabidopsis plants were downregulated, while the transcript abundance of SlTIP2;2 was increased in the shoots after salinity stress. The authors interpreted the downregulation of SlTIP2;2 expression in the roots as to reduce water efflux and prevent the deleterious effects of excessive salt loading. Similarly, the transcripts of TIPs were sharply upregulated in the leaves but downregulated in the roots of A. canescens under NaCl treatment (Guo et al. 2019), which are likely to be involved in the transport of water into salt bladders under salinity facilitating salt secretion and maintenance of water balance in leaves. This osmotic balance results from the accumulation of Na+ in leaf tissues and salt bladders (Guo et al. 2019). Consistently, expression of OsAQP gene in rice, encodes TIP, was upregulated by high salinity in leaves, but downregulated in roots, indicative of its essential role in the defense of rice against salinity stress (Liang et al. 2013). The study suggests that OsAQP may contribute to osmotic adjustment by accelerating the flow of water in leaves, while decreasing the expression level of OsAQP in roots may reduce salt uptake under salinity stress. It is also possible that the decrease in the expression of aquaporins might be compensated for by a greater activity of aquaporin proteins in response to salinity (Coelho et al. 2021). An et al. (2017) also demonstrated that overexpression of PgTIP1 in soybeans promotes the upregulation of NHX, SOS1, CAT1, and APX1 and thus contributing to ion compartmentalization in the vacuole and antioxidant defense under salinity (Table 3). Conversely, although TIP genes are associated with plant tolerance to abiotic stresses, overexpression of GsTIP2;1 in Arabidopsis thaliana depressed tolerance to salinity stress (Wang et al. 2011b). Further, TaTIP2;2 acts as a negative regulator of the salinity stress response as salinity stress downregulated it (Xu et al. 2013). Taken together, AQPs seem to play complex and diverse roles in the response of plants to salinity, which may be dependent on specific isoforms, organ, duration, and degree of stress. A better understanding of the AQP family members and their functions in plant response to saline soil is, however, still required.
Vacuolar ATP-binding Cassette (ABC) Transporters and Tolerance to Saline Conditions
Types and Functions of ABC Transporters
The ATP-binding cassette (ABC) transporters belong to a large group of protein families involved in import/export of various metabolites inside the cell system (Dahuja et al. 2021). Most ABC systems are transport proteins that couple the free energy of ATP hydrolysis to the translocation of solutes across a biological membrane (Licht and Schneider 2011; Dhara and Raichaudhuri 2021). ABC transporters therefore constitute more than 50% of all energy-dependent transport proteins, which are encoded in the genomes of almost all organisms (Nagata et al. 2008). ABC transporters were initially shown to play an important role in cell detoxification by removing the unwanted chemicals from the cytosol, but currently their function validated to be involved in different biological processes in plants and was mostly localized in the cellular membranes (Dahuja et al. 2021; Dhara and Raichaudhuri 2021). The functions of ABC transporters are diverse, including heavy metal sequestration, chlorophyll catabolite transport, pumps of glutathione-S conjugates, pathogen response, surface lipid deposition, inorganic acid transport, cell wall monomers, phytate accumulation in seeds, and transport of the phytohormones (Kang et al. 2011; Shi et al. 2020; Dahuja et al. 2021; Dhara and Raichaudhuri 2021). In addition, ABC systems can fulfill essential functions, such as evoking distinct signaling cascades and ion channel regulation, while novel and unexpected functions and substrates of these proteins are still waiting to be elucidated (Wanke and Kolukisaoglu 2010). ABC transporters also play an important role in organ growth, plant nutrition, plant development, plant interaction with its environment, and accumulation of minerals, lipids, and cutin in seeds (Kang et al. 2011). In response to saline conditions, ABC transporters have been shown to transport various secondary metabolites, such as terpenoids, quinones, alkaloids, and polyphenols, which are predicted to function as protectants for different biotic and abiotic stresses (Dahuja et al. 2021). Based on the domain structure, the ABC transporters in Arabidopsis have been classified into eight sub-families designated as A, B, C, D, E, F, G, and I (Gräfe and Schmitt 2020). For example, ABCB transporters are critical for polar auxin transport and distribution (Hao et al., 2020). Also, all ABCC genes showed significant increases during the seed development stages but only ABCC8 and ABCC11 showed significant increases during fruit development in strawberry (Shi et al. 2020). Interestingly, overexpression of FvABCC11 in Arabidopsis partially restored seedling development under Cd treatment, indicating that FvABCC11 increased Cd tolerance (Shi et al. 2020).
Expression of ABC Transporter Genes Under Salinity Stress
Little information is published about the involvement of the tonoplast-localized ABC transporters in plant responses and tolerance to saline soils. For example, Zhang et al. (2020a) revealed that ABC genes possess non-tissue specificity with 15 differentially expressed genes exhibited diverse expression responses to stress treatments, including salinity stress. The authors indicated that the ABCB and ABCG sub-families function in the response to abiotic stress in barley, which confirms the possible potential role of ABC transporters in response to stress. Also, An et al. (2019) reported that 15 different BhABC genes were identified and validated to be involved in modulating the tolerance against salinity and drought stresses. Among them, ABCB and ABCG were reported to be the most active in responses to hormones and abiotic stress (Table 3). Under salinity stress, upregulation of an ABC transporter in cotton roots enhanced plant tolerance through sequestration of Na+ into the vacuole, indicative of their role in salinity response and tolerance (Li et al. 2015). Also, among proteins identified in the tonoplast of the mangrove Avicennia officinalis, ABC transporters, K+ transporters, and AQPs were predominant, suggesting their role in Na+ sequestration, ion homeostasis, and turgor regulation (Krishnamurthy et al. 2014). Furthermore, ABC transporters (AtATH14 and AtATH15) were found to be responsive to salinity stress in Arabidopsis as their transcript levels were changed (Maathuis et al. 2003), suggesting that AtATH14 and AtATH15 might be implicated in salinity stress response and tolerance. In accordance, ABC transporter AtMRP5 altered Na+/K+ homeostasis and evoked salinity stress response in Arabidopsis (Lee et al. 2004). Additionally, overexpression of AtABCG36/AtPDR8 in Arabidopsis resulted in improving tolerance to salinity by producing higher shoot biomass, reduced Na+ content, and less chlorotic leaves than the wild-type plants (Table 3, Kim et al. 2010). Higher members of ABCG proteins in rice genome were also reported in response to salinity stress, indicative of possible role in improving response and tolerance to salinity stress (Saha et al. 2015). Nguyen et al. (2014) demonstrated similarly that nine rice ABC transporters are differentially regulated by drought stress in root, suggesting biological function. In the same trend, salinity stress has been shown to control expression of Prb (PROLINE BETAINE-INDUCIBLE ABC transporter) and to increase Prb transport activity in Sinorhizobium meliloti (Alloing et al. 2006); the importance of that is proline betaine acts as an osmoprotectant, which might be extrapolated to higher plants in response to saline environments. In bacteria, the expression of salinity-tolerant genes also effectively improve their survival in saline soil, and ABC transporter gene is one of these salinity-tolerant genes contributed to salinity tolerance of Bacillus sp. strain “SX4” obtained from salinized greenhouse soil (Zhang et al. 2021). We speculate that ABC transporters may participate in maintenance of osmotic balance by regulating the concentration of Na+ in the cell and facilitate the uptake of osmoprotectants, transport of secondary metabolites, and phytohormones under high salinity. Therefore, it is likely that elucidation of the mechanistic basis of any given plant process will necessitate consideration of at least one ABC transporter. However, our knowledge about the association of ABC proteins with plant tolerance to saline environment is rather scarce and remains to be further clarified and explored. Also, the ABC gene family has not been identified and analyzed in most crop species, which need more future research.
Vacuolar Myo-inositol Transporter (INT)
Further tonoplast-located transporter that has been found to be involved in apple salinity tolerance is the tonoplast-localized myo-inositol transporter 1 (INT1, Hu et al. 2017, 2020). INT belongs to the monosaccharide transporter (MST) superfamily and has shown to import myo-inositol into the cytoplasm from the vacuoles in Arabidopsis (Schneider et al. 2008). In addition, exogenous application and overexpression of myo-inositol alleviated salinity-induced stress and enhanced tolerance to NaCl stress in different plant species (Sambe et al. 2015; Hu et al. 2017, 2020). Interestingly, Hu et al. (2020) reported that tonoplast MdINT1 plays an important role in myo-inositol accumulation, and its overexpression conferred transgenic apple salinity tolerance by regulating the antioxidant systems, ion homeostasis, and accumulation of compatible solutes (i.e., sucrose, glucose, sorbitol). It is important to note that overexpression of MdINT1 reduced Na+ accumulation and increased K+ concentration in salinity-stressed apple plants. It is clear that tonoplast MdINT1 regulates other genes implicated in salinity tolerance. Although little information is known about INT1 and requires further experimental justifications, INT1 might be a potential target in biotechnology and agriculture.
Concluding Remarks
We presented different lines of evidence confirming the key role of vacuolar membrane transport systems in adaptation of numerous plant species to saline conditions. In particular, vacuolar H+-ATPase, H+-PPase, and Na+/H+ antiporters have been demonstrated to contribute to one critical salinity tolerance mechanism, i.e., Na+ sequestration into the vacuoles under saline conditions. In support, overexpression of a gene encoding one of these transport proteins improved salinity tolerance of the transgenic plants in response to high salinity. This molecular progress in the field of salinity stress will certainly unmask the cellular mechanisms that underlie tolerance, but the road to engineering such tolerance into sensitive crops will be long since salinity stress resistance is a complex multigenic trait. Therefore, due to this polygenic nature of salinity tolerance trait, transgene pyramiding of more than one gene is required for developing genotypes with better and strong salinity tolerance and productivity and should gain more attention and focus in future research. Additionally, an important component of crop salinity tolerance is not only the ability to grow under salinity stress but also to produce high grain yield. In fact, most studies in the salinity stress issue are with a limited focus on evaluating yield traits. The field trial is therefore necessary to validate the feasibility of this strategy for agricultural use. Field performance is also addressing the question of whether the manipulation of these vacuolar transport systems is associated with a significant cost in yield, i.e., yield penalty. Furthermore, the strategies suggested by Shabala et al. (2020) concerning the regulation of the activity of tonoplast SV and FV channels need to be strongly considered in the future research in order to regulate the activity of tonoplast SV and FV channels to improve energy use efficiency and hence salinity tolerance in plants. Investigations on plant physiological and molecular mechanisms of tolerance to salinity have mostly focused on Na+ toxicity and adaptations, while Cl− impact on plants caused by salinity stress remains under-investigated. CLCs are critical for Cl− and NO3− homeostasis and thus adjustment of cellular turgor, stomatal movement, and signal transduction under salinity stress. This issue therefore is a promising and important area for future research that remains to be further studied and elucidated in order to unmask an important component of salinity puzzle. Although little is known about the regulation and function of vacuolar CAXs, ABC transporters, and aquaporins in plants under salinity stress, they are potential candidates to be used as one of the mechanisms to protect plants under salinity stress and should be more explored and focused physiologically and genetically in order to engineer them in crop plants. It is obvious that vacuolar transport systems should be targeted in breeding programs and transgenic approaches. In any case, comprehensive and intensive field performance and high-yield analysis of transgenic plants under salinity stress should be always carried out and focused following these approaches in order to promote and validate their application for agricultural use in marginal regions.
References
Abdul Kader M, Seidel T, Golldack D, Lindberg S (2006) Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J Exp Bot 57:4257–4268
Adem GD, Roy SG, Zhou M, Bowman JA, Shabala S (2014) Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol 14:113
Adem GD, Roy SJ, Plett DC, Zhou MX, Bowman JP, Shabala S (2015) Expressing AtNHX1 in barley (Hordium vulgare L.) does not improve plant performance under saline conditions. Plant Growth Regul 77:289–297
Adem GD, Roy SJ, Huang Y, Chen Z, Wang F, Zhou M, Bowman JP, Holford P, Shabala S (2017) Expressing Arabidopsis thaliana V-ATPase subunit C in barley (Hordeum vulgare) improves plant performance under saline condition by enabling better osmotic adjustment. Funct Plant Biol 44:1147–1159
Afzal Z, Howton TC, Sun Y, Mukhtar MS (2016) The roles of aquaporins in plant stress responses. J Dev Biol 4:9
Aghaei K, Komatsu S (2013) Crop and medicinal plants proteomics in response to salt stress. Front Plant Sci 4:1–9
Alexopoulos AA, Assimakopoulou A, Panagopoulos P, Bakea M, Vidalis N, Karapanos IC, Petropoulos SA (2021) Impact of salinity on the growth and chemical composition of two underutilized wild edible greens: Taraxacum officinale and Reichardia picroides. Horticulturae 7:160
Al-Harrasi I, Jana GA, Patankar HV, Al-Yahyai R, Rajappa S, Kumar PP, Yaish MW (2020) A novel tonoplast Na+/H+ antiporter gene from date palm (PdNHX6) confers enhanced salt tolerance response in Arabidopsis. Plant Cell Rep 39:1079–1093
Alloing G, Travers I, Sagot B, Rudulier DL, Dupont L (2006) Proline betaine uptake in Sinorhizobium meliloti: characterization of Prb, an Opp-like ABC transporter regulated by both proline betaine and salinity stress. J Bacteriol 118:6308–6317
Amin USM, Biswas S, Elias SM, Razzaque S, Haque T, Malo R, Seraj ZI (2016) Enhanced salt tolerance conferred by the complete 2.3 kb cDNA of the rice vacuolar Na+/H+ antiporter gene compared to 1.9 kb coding region with 5’ UTR in transgenic lines of rice. Front Plant Sci 7:14
An J, Hu Z, Che B, Chen H, Yu B, Cai W (2017) Heterologous expression of Panax ginseng PgTIP1 confers enhanced salt tolerance of soybean cotyledon hairy roots, composite, and whole plants. Front Plant Sci 8:1232
An L, Ma Q, Du J, Yu M, Li F, Luan J, Jiang J, Li H (2019) Preliminary classification of the ABC transporter family in Betula halophila and expression patterns in response to exogenous phytohormones and abiotic stresses. Forests 10:722
Angeli A, Zhang J, Meyer S, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4:1804
Anjaneyulu E, Reddy PS, Sunita MSL, KaviKishor PB, Meriga B (2014) Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H+-pyrophosphatase gene (SbVPPase) from Sorghum bicolor. J Plant Physiol 171:789–798
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509
Baetz U, Eisenach C, Tohge T, Martinoia E, De Angeli A (2016) Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol 172:1167–1181
Baghour M, Galvez FJ, Sanchez ME, Aranda MN, Venema K, Rodrıguez-Rosales MP (2019) Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant Physiol Biochem 135:77–86
Baisakh N, Rao MV, Rajasekaran K, Subudhi P, Janda J, Galbraith D, Vanier C, Pereira A (2012) Enhanced salt stress tolerance of rice plants expressing a vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from the halophyte grass Spartina alterniflora Loisel. Plant Biotechnol J 10:453–464
Banjara M, Zhu L, Shen G, Payton P, Zhang H (2012) Expression of an Arabidopsis sodium/proton antiporter gene (AtNHX1) in peanut to improve salt tolerance. Plant Biotechnol Rep 6:59–67
Bao A, Wang SM, Wu GQ, Xi JJ, Wang CM (2008) Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci 176:232–240
Bao A, Wang SM, Wu GQ, Xi JJ, Zhang JL, Wang CM (2009) Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci 176:232–240
Bao A, Wang Y, Xi J, Liu C, Zhang J, Wang S (2013) Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation. Funct Plant Biol 41:203–214
Bao A, Du B, Touil L, Kang P, Wang Q, Wang S (2016) Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotech J 14:964–975
Barkla BJ, Pantoja O (1996) Physiology of ion transport across the tonoplast of higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:159–184
Barkla BJ, Zingarelli L, Blumwald E, Smith JAC (1995) Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol 109:549–556
Barkla BJ, Vera-Estrella R, Hernandez-Coronado M, Pantoja O (2009) Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance. Plant Cell 21:4044–4058
Barragán V, Leidi EO, Andrés Z, Rubio L, DeLuca A, Fernández JA (2012) Ion exchangers NHX1and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24:1127–1142
Barros NLF, Marques DN, Tadaiesky LBA, de Souza CRB (2021) Halophytes and other molecular strategies for the generation of salt-tolerant crops. Plant Phsiol Biochem 162:581–591
Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol 22:1–6
Bassil E, Tajima H, Liang YC, Ohto M, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23:3482–3497
Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63:5727–5740
Bassil E, Zhang S, Gong H, Tajima H, Blumwald E (2019) Cation specificity of vacuolar NHX-type cation/H+ antiporters. Plant Physiol 179:616–629
Batelli G, Verslues PE, Agius F, Qiu QS, Zhu JK (2007) SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol Cell Biol 27:7781–7790
Bezerra-Neto P, Araujo FC, Ferreira-Neto JRC, Silva MD, Pandolfi V, Aburjaile FF, Sakamoto T, Silva RLO, Kido EA, Amorim LLB, Ortega JM, Benko-Iseppon AM (2019) Plant aquaporins: diversity, evolution and biotechnological applications. Curr Protein Pept Sci 20:368–395
Bhaskaran S, Savithramma DI (2011) Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+- pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot 62:5561–5570
Biradar H, Karan R, Subudhi PK (2018) Transgene pyramiding of salt responsive protein 3–1 (SaSRP3–1) and SaVHAc1 from Spartina alterniflora L. enhances salt tolerance in rice. Front Plant Sci 9:1304
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1456:140–151
Bonales-Alatorre E, Pottosin I, Shabala L, Chen Z, Zeng F, Jacobsen S (2013a) Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa. Int J Mol Sci 14:9267–9285
Bonales-Alatorre E, Shabala S, Chen ZH, Pottosin I (2013b) Reduced tonoplast FV and SV channels activity is essential for conferring salinity tolerance in a facultative halophyte, Chenopodium quinoa. Plant Physiol 162:940–952
Bonza MC, De Michelis MI (2010) The plant Ca2+-ATPase repertoire: biochemical features and physiological functions. Plant Biol 13:421–430
Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58:301–308
Bulle M, Yarra R, Abbagani S (2016) Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol Breed 36:36
Chakraborty K, Sairam RK, Bhattacharya RC (2012) Differential expression of salt overly sensitive pathway genes determines salinity stress tolerance in Brassica genotype. Plant Physiol Biochem 51:90–101
Chen S, Li J, Fritz E, Wang S, Huttermann A (2002) Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress. For Ecol Manag 168:217–230
Chen H, An R, Tang JH, Cui XH, Hao FS, Chen J, Wang XC (2007) Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol Breed 19:215–225
Chen Y, Li L, Zong J, Chen J, Guo H, Guo A, Liu J (2015) Heterologous expression of the halophyte Zoysia matrella H+-pyrophosphatase gene improved salt tolerance in Arabidopsis thaliana. Plant Physiol Biochem 91:49–55
Cheng X, Wang Y, Bao A, Wang S (2011) Overexpression of AVP1 enhanced salt and drought tolerance of transgenic lotus corniculatus L. Plant Physiol Commun 46:808–816 ((in Chinese))
Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM (2012) Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol 160:1293–1302
Churchill KA, Sze H (1984) Anion-sensitive, H+-pumping ATPase of oat roots: direct effects of Cl−, NO3−, and a disulfonic stilbene. Plant Physiol 76:490–497
Coelho MRV, Rivas R, Ferreira-Neto JRC, Bezerra-Neto JP, Pandolfi V, Benko-Iseppon AM, Santos MG (2021) Salt tolerance of Calotropis procera begins with immediate regulation of aquaporin activity in the root system. Physiol Mol Biol Plants 27:457–468
Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AAR (2011) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23:240–257
Cosentino C, Fischer-Schliebs E, Bertl A, Thiel G, Homann U (2010) Na+⁄H+ antiporters are differentially regulated in response to NaCl stress in leaves and roots of Mesembryanthemum crystallinum. New Phytol 186:669–680
Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S (2011) Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant Cell Environ 34:947–961
Dahuja A, Kumar RR, Sakhar A, Watts A, Singh B, Goswami S, Sachdev A, Praveen S (2021) Role of ATP-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses. Physiol Plant 171:785–801
Debez A, Saadaoui D, Ramani B, Ouerghi Z, Koyro H, Huchzermeyer B, Abdelly C (2006) Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ Exp Bot 57:285–295
Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytol 220:49–69
Dhara A, Raichaudhuri A (2021) ABCG transporter proteins with beneficial activity on plants. Phytochemistry 184:112663
Diédhiou CJ, Golldack D (2006) Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci 170:793–800
Dietz KJ, Tavakoli N, Kluge C, Mimura T, Sharma SS, Harris GC, Chardonnens AN, Golldack D (2001) Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J Exp Bot 52:1969–1980
Dindas J, Dreye I, Huang S, Hedrich R, Roelfsema MRG (2021) A voltage-dependent Ca2+ homeostat operates in the plant vacuolar membrane. New Phytol 230:1449–1460
Dong X, Sun L, Guo J, Liu L, Han G, Wang B (2021b) Exogenous boron alleviates growth inhibition by NaCl stress by reducing Cl− uptake in sugar beet (Beta vulgaris). Plant Soil 464:423–439
Dong J, Liu C, Wang Y, Zhao Y, Ge D, Yuan Z (2021) Genome-wide identification of the NHX gene family in Punica granatum L. and their expressional patterns under salt stress. Agronomy 11:264
Dreyer I, Sussmilch FC, Fukushima K, Riadi G, Becker D, Schultz J, Hedrich R (2021) How to grow a tree: plant voltage-dependent cation channels in the spotlight of evolution. Trends Plant Sci 26:41–52
Eljebbawi A, Guerrero YCR, Dunand C, Estevez JM (2021) Highlighting reactive oxygen species as multitaskers in root development. iScience 24:101978
Evans MJ, Choi WG, Gilroy S, Morris RJ (2016) A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol 171:1771–1784
Fan W, Denga G, Wang H, Zhang H, Zhang P (2015) Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiol Plant 154:560–571
FAO (2009) High level expert forum—how to feed the world in 2050. Economic and Social Development, Food and Agricultural Organization of the United Nations, Rome, Italy
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot 115:419–431
Fukuda A, Chiba K, Maeda M, Nakamura A, Maeshima M, Tanaka Y (2004) Effect of salt and osmotic stresses on the expression of genes for the vacuolar H+-pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter from barley. J Exp Bot 55:585–594
Fukuda A, Nakamura A, Hara N, Toki S, Tanaka Y (2011) Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta 233:175–188
Galvez FJ, Baghour M, Hao GP, Cagnac O, Rodriguez-Rosales MP, Venema K (2012) Expression of LeNHX isoforms in response to salt stress in salt sensitive and salt tolerant tomato species. Plant Physiol Biochem 51:109–115
Gao C, Wang Y, Jiang B, Liu G, Yu L, Wei Z, Yang C (2011) A novel vacuolar membrane H+-ATPase c subunit gene (ThVHAc1) from Tamarix hispida confers tolerance to several abiotic stresses in Saccharomyces cerevisiae. Mol Biol Rep 38:957–963
Gao C, Zhao Q, Jiang L (2015) Vacuoles protect plants from high magnesium stress. Proc Natl Acad Sci (USA) 10:2931–2932
Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci (USA) 96:1480–1485
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL (2001) Drought-and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci (USA) 98:11444–11449
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Gouiaa S, Khoudi H (2015) Co-expression of vacuolar Na+/H+ antiporter and H+-pyrophosphatase with an IRES-mediated dicistronic vector improves salinity tolerance and enhances potassium biofortification of tomato. Phytochem 117:537–546
Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K (2012) Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Mol Biol 79:137–155
Gräfe K, Schmitt L (2020) The ABC transporter G subfamily in Arabidopsis thaliana. J Exp Bot 72:92–106
Graus D, Konrad KR, Bemm F, Nebioglu MG, Lorey C, Duscha K, Güthoff T, Herrmann J, Ferjani A, Cuin TA, Roelfsema MRG, Schumacher K, Neuhaus HE, Marten I, Hedrich R (2018) High V-PPase activity is beneficial under high salt loads, but detrimental without salinity. New Phytol 219:1421–1432
Gu R, Liu Q, Pei D, Jian X (2004) Understanding saline and osmotic tolerance of Populus euphratica suspended cells. Plant Cell Tissue Organ Cult 78:261–265
Guo H, Zhang L, Cui Y, Wang S, Bao A (2019) Identification of candidate genes related to salt tolerance of the secretohalophyte Atriplex canescens by transcriptomic analysis. BMC Plant Biol 19:213
Guo Q, Tian XX, Mao PC, Meng L (2020) Overexpression of Iris lactea tonoplast Na+/H+ antiporter gene IlNHX confers improved salt tolerance in tobacco. Biol Plant 64:50–57
Gupta A, Shaw BP, Roychoudhury A (2021) NHX1, HKT, and monovalent cation transporters regulate K+ and Na+ transport during abiotic stress. In: Deshmukh R, Roychoudhury HA, Tripathi D (eds) Transporters and plant osmotic stress. Elsevier, London, pp 1–27
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014:701596
Hamada A, Shono M, Xia T, Ohta M, Hayashi Y, Tanaka A, Hayakawa T (2001) Isolation and characterization of a Na+/H+ antiporter gene from the halophyte Atriplex gmelini. Plant Mol Biol 46:35–42
Hao P, Xia J, Liu J, Donato M, Pakula K, Bailly A, Jasinski M, Geisler M (2020) Auxin-transporting ABC transporters are defined by a conserved D/E-P motif regulated by a prolylisomerase. J Biol Chem 295:13094–13105
Hasanuzzaman M, Hossain M, de Silva JT (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateswarlu B, Shanker AK, Shanker C, Maheswari M (eds) Crop stress and its management: perspectives and strategies. Springer, The Netherlands, pp 261–315
He Y, Fu J, Yu C, Wang X, Jiang Q, Hong J (2015) Increasing cyclic electron flow is related to Na+ sequestration into vacuoles for salt tolerance in soybean. J Exp Bot 66:6877–6889
He X, Huang X, Shen Y, Huang Z (2014) Wheat V-H+-ATPase subunit genes significantly affect salt tolerance in Arabidopsis thaliana. PLoS One 9:e86982
Hedrich R, Sauer N, Neuhaus HE (2015) Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins. Curr Opin Plant Biol 25:3–70
Hedrich R, Mueller TD, Becker D, Marten I (2018) Structure and function of TPC1 vacuole SV channel gains shape. Mol Plants 11:764–775
Henderson SW, Baumann U, Blackmore DH, Walker AR, Walker RR, Gilliham M (2014) Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biol 14:273
Hilleary R, Paez-Valencia J, Vens C, Toyota M, Palmgren M, Gilroy S (2020) Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. PNAS 117:18849–18857
Hocking B, Conn SJ, Manohar M, Xu B, Athman A, Stancombe MA, Webb AR, Hirschi KD, Gilliham M (2017) Heterodimerization of Arabidopsis calcium/proton exchangers contributes to regulation of guard cell dynamics and plant defense responses. J Exp Bot 68:4171–4183
Hong-Hermesdorf A, Brüx A, Grüber A, Grüber G, Schumacher K (2006) WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett 580:932–939
Hossain MM, Imran S, Islam MS, Abdul Kader M, Imtiaz Uddin M (2017) Expressional analysis of OsNHX1, OsNHX2, OsSOS1 and OsDREB transporters in salt tolerant (FR13A) and salt sensitive rice (brri dhan29) induced by salinity stress. IOSR J Agric Vet Sci 10:64–70
Hu R, Zhu Y, Wei J, Chen J, Shi H, Shen G (2017) Overexpression of PP2A-C5 that encodes the catalytic subunit 5 of protein phosphatase 2A in Arabidopsis confers better root and shoot development under salt conditions. Plant Cell Environ 40:150–164
Hu L, Zhou K, Yang S, Liu Y, Li Y, Zhang Z, Zhang J, Gong X, Ma F (2020) MdINT1 enhances apple salinity tolerance by regulating the antioxidant system, homeostasis of ions, and osmosis. Plant Physiol Biochem 154:698–698
Huang Y, Guan C, Liu Y, Chen B, Yuan S, Cui X, Zhang Y, Yang F (2017) Enhanced growth performance and salinity tolerance in transgenic switchgrass via overexpressing vacuolar Na+ (K+)/H+ antiporter gene (PvNHX1). Front Plant Sci 8:458
Huertas R, Olias R, Eljakaoui Z, Gálvez FJ, Li J, de Morales PA, Belver A, Rodríguez-Rosales MP (2012) Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ 35:1467–1482
Isayenkov S, CharlesIsner J, Maathuis FJM (2010) Vacuolar ion channels: roles in plant nutrition and signaling. FEBS Lett 84:1982–1988
Jaarsma R, de Boer AH (2018) Salinity tolerance of two potato cultivars (Solanum tuberosum) correlates with differences in vacuolar transport activity. Front Plant Sci 9:737
Janda T, Darko E, Shehata S, Kovacs V, Pal M, Szalai G (2016) Salt acclimation processes in wheat. Plant Physiol Biochem 101:68–75
Jaślan D, Dreyer I, Lu J, O’Malley R, Dindas J, Marten I, Hedrich R (2019) Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat Commun 10:2659
Jha B, Mishra A, Jha A, Joshi M (2013) Developing transgenic jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline wasteland. PLoS One 8:e71136
Jia Q, Zheng C, Sun S, Amjad H, Liang K, Lin W (2018) The role of plant cation/proton antiporter gene family in salt tolerance. Biol Plant 62:617–629
Jiang X, Leidi EO, Pardo JM (2010) How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav 5:792–795
Jossier M, Kroniewicz L, Dalmas F, Le Thiec D, Ephritikhine G, Thomine S (2010) The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J 64:563–576
Kaldenhoff R, Fischer M (2006) Functional aquaporin diversity in plants. Acta Biochim Biophys 1758:11334–21141
Kang J, Park J, Choi H, Burla B, Kretzschmar T, Lee Y, Martinoia E (2011) Plant ABC Transporters. Arabidopsis Book 9:153. https://doi.org/10.1199/tab.0153
Khan K, Agarwal P, Shanware A, Sane VA (2015) Heterologous expression of two jatropha aquaporins imparts drought and salt tolerance and improves seed viability in transgenic Arabidopsis thaliana. PLoS ONE 10:e0128866
Kim DY, Jina JY, Alejandrob S, Martinoiaa E, Lee Y (2010) Overexpression of AtABCG36 improves drought and salt stress resistance in Arabidopsis. Physiol Plant 139:170–180
Kimura S, Hunter K, Vaahtera L, Tran HC, Citterico M, Vaattovaara A, Wilkens MMT (2020) CRK2 and C-terminal phosphorylation of NADPH oxidase RBOHD regulate reactive oxygen species production in Arabidopsis. Plant Cell 32:1063–1080
Klychnikov OI, Li KW, Lill H, de Boer AH (2007) The V-ATPase from etiolated barley (Hordeum vulgare L.) shoots is activated by blue light and interacts with 14-3-3 proteins. J Exp Bot 58:1013–1123
Koselski M, Trebacz K, Dziubinska H (2019) The role of vacuolar ion channels in salt stress tolerance in the liverwort Conocephalum conicum. Acta Physiol Plant 41:110
Krishnamurthy P, Tan XF, Lim TK, Lim T, Kumar PP, Loh C, Lin Q (2014) Proteomic analysis of plasma membrane and tonoplast from the leaves of mangrove plant Avicennia officinalis. Proteomics 14:2545–2557
Kurowska MM (2021b) Aquaporins in cereals - important players in maintaining cell homeostasis under abiotic stress. Genes 12:477
Kurowska MM (2021) TIP aquaporins in plants: role in abiotic stress tolerance. In: Fahad S, Saud S, Chen Y (eds) Abiotic stress in plants. IntechOpen, Croatia, pp 1–21
Lee EK, Kwon M, Ko J, Yi H, Hwang MG, Chang S, Cho MH (2004) Binding of sulfonylurea by AtMRP5, an Arabidopsis multidrug resistance-related protein that functions in salt tolerance. Plant Physiol 134:528–538
Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61:495–506
Li WYF, Wong FL, Tsai SN, Phang TH, Shao G, Lam HM (2006) Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY) -2 cells. Plant Cell Environ 29:1122–1137
Li G, Peng Y, Yu X, Zhang M, Cai W, Sun W, Su W (2008) Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J Plant Physiol 165:1879–1888
Li ZG, Baldwin CM, Hu Q, Liu H, Luo H (2010) Heterologous expression of Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Cell Environ 33:272–289
Li X, Guo C, Gu J, Duan W, Zhao M, Ma C, Du X, LuW XK (2014a) Overexpression of VP, a vacuolar H+-pyrophosphatase gene in wheat (Triticum aestivum L.), improves tobacco plant growth under Pi and N deprivation, high salinity, and drought. J Exp Bot 65:683–696
Li G, Santoni V, Maurel C (2014b) Plant aquaporins: roles in plant physiology. Acta Gen Subj 1840:1574–1582
Li N, Wang X, Ma B, Du C, Zheng L, Wang Y (2017) Expression of a Na+/H+ antiporter RtNHX1 from a recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana. J Plant Physiol 218:109–120
Li W, Zhao F, Fang W, Xie D, Hou J, Yang X (2015) Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front Plant Sci 6:732
Liang W, Li L, Zhang F, Liu Y, Li M, Shi H, Li H, Shang F, Lou C, Lin Q, Li J, Yang X (2013) Effects of abiotic stress, light, phytochromes and phytohormones on the expression of OsAQP, a rice aquaporin gene. Plant Growth Regul 69:21–27
Liang M, Lin M, Lin Z, Zhao L, Zhao G, Li Q, Yin X (2015) Identification, functional characterization, and expression pattern of a NaCl-inducible vacuolar Na+/H+ antiporter in chicory (Cichorium intybus L.). Plant Growth Regul 75:60–614
Licht A, Schneider E (2011) ATP binding cassette systems: structures, mechanisms, and functions. Cent Eur J Biol 6:785–801
Liu L, Fan X, Wang F, Wang N, Dong Y, Liu X, Yang J, Wang Y, Li H (2013) Coexpression of ScNHX1 and ScVP in transgenic hybrids improves salt and saline-alkali tolerance in alfalfa (Medicago sativa L.). J Plant Growth Regul 32:1–8
Liu W, Yuan X, Zhang Y, Xuan Y, Yan Y (2014) Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Physiol Plant 36:2183–2193
Liu X, Cai S, Wang G, Wang F, Dong F, Mak M, Holford P, Ji J, Salih A, Zhou M, Shabala S, Chen Z (2017) Halophytic NHXs confer salt tolerance by altering cytosolic and vacuolar K+ and Na+ in Arabidopsis root cell. Plant Growth Regul 82:333–351
Liu J, Hu J, Li Y, Li G, Wu H (2021) Calcium channels and transporters in plants under salinity stress. In: Upadhyay SK (ed) Calcium transport elements in plants. Academic Press, London, pp 157–169
Low R, Rockel B, Kirsch M, Ratajczak R, Hörtensteiner S, Martinoia E, Lüttge U, Rausch T (1996) Early salt stress effects on the differential expression of vacuolar H+-ATPase genes in roots and leaves of Mesembryanthemum crystallinum. Plant Physiol 110:259–265
Lu W, Guo C, Li X, Duan W, Ma C, Zhao M, Gu J, Du X, Liu Z, Xiao K (2014) Overexpression of TaNHX3, a vacuolar Na+/H+ antiporter gene in wheat, enhances salt stress tolerance in tobacco by improving related physiological processes. Plant Physiol Biochem 76:17–28
Lv S, Zhang K, Gao Q (2008) Overexpression of an H+-PPase gene from Thellungiella halophila in cotton enhances salt tolerance and improves growth and photosynthetic performance. Plant Cell Physiol 49:1150–1164
Lv S, Jiang P, Nie L, Chen X, Tai F, Wang D, Fan P, Feng J, Bao H, Wang J, Li Y (2015) H+-pyrophosphatase from Salicornia europaea confers tolerance to simultaneously occurring salt stress and nitrogen deficiency in Arabidopsis and wheat. Plant Cell Environ 38:2433–2449
Lv S, Jiang P, Tai F, Wang D, Feng J, Fan P, Bao H, Li Y (2017) The V-ATPase subunit A is essential for salt tolerance through participating in vacuolar Na+ compartmentalization in Salicornia europaea. Planta 246:1177–1187
Maach M, Baghour M, Akodad M, Gálvez FJ, Sánchez ME, Aranda MN, Venema K, Rodríguez-Rosales MP (2020) Overexpression of LeNHX4 improved yield, fruit quality and salt tolerance in tomato plants (Solanum lycopersicum L.). Mol Biol Rep 47:4145–4153
Maathuis FJ, Filatov V, Herzyk PC, Krijger GB, Axelsen K, Chen S, Green BJ, Li Y, Madagan KL, Sanchez-Fernandez R (2003) Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 35:675–692
Maeshima M (2001) Tonoplast transporters: organization and function. Annu Rev Plant Physiol Plant Mol Biol 52:469–497
Mansour MMF (2014) The plasma membrane transport systems and adaptation to salinity. J Plant Physiol 171:1787–1800
Mansour MMF, Ali EF (2017) Glycinebetaine in saline conditions: an assessment of the current state of knowledge. Acta Physiol Plant 39:56
Mansour MMF, Stadelmann EJ (1994) NaCl-induced changes in protoplasmic characteristics of Hordeum vulgare cultivars differing in salt tolerance. Physiol Plant 91:389–394
Mansour MMF, Lee-Stadelmann OY, Stadelmann EJ (1993) Solute potential and cytoplasmic viscosity in Triticum aestivum and Hordeum vulgare under salt stress. A comparison of salt resistant and salt sensitive lines and cultivars. J Plant Physiol 142:623–628
Mansour MMF, van Hasselt PR, Kuiper PJC (1998) Ca2+, Mg2+-ATPase activities in winter wheat root plasma membranes as affected by NaCl stress during growth. J Plant Physiol 153:181–187
Mansour MMF, Ali EF, Salama KHA (2019) Does seed priming play a role in regulating reactive oxygen species under saline conditions? In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Wiley and Sons, New Jersey, pp 437–488
Mansour MMF (1995) NaCl alteration of plasma membrane of Allium cepa epidermal cells. Alleviation by calcium. J Plant Physiol 145:726–730
Mansour MMF, Emam MM, Salama KHA, Morsy AA (2021) Sorghum under saline conditions: responses, tolerance mechanisms, and management strategies. Planta 254:24
Maron L (2019) From foe to friend: the role of chloride as a beneficial macronutrient. Plant J 99:813–814
Martínez-Alcántara B, Martínez-Cuenca MR, Quinones A, Iglesias DJ, Primo-Millo E, Forner-Giner MA (2015) Comparative expression of candidate genes involved in sodium transport and compartmentation in citrus. Environ Exp Bot 111:52–62
Martinez-Ballesta MC, Carvajal M (2014) New challenges in plant aquaporin biotechnology. Plant Sci 217–218:71–77
Martinoia E, Maeshima M, Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58:83–102
Martins CPS, Neves DM, Cidade LC, Mendes AFS, Silva DC, Almeida AF, Filho MAC, Gesteira AS, Soares-Filho WS, Costa C (2017) Expression of the citrus CsTIP2;1 gene improves tobacco plant growth, antioxidant capacity and physiological adaptation under stress conditions. Planta 245:951–963
Miranda RS, Mesquita RO, Costa JH, Alvarez-Pizarro JC, Prisco JT, Gomes-Filho E (2017) Integrative control between proton pumps and SOS1 antiporters in roots is crucial for maintaining low Na+ accumulation and salt tolerance in ammonium-supplied Sorghum bicolor. Plant Cell Physiol 58:522–536
Mishra S, Alavilli H, Lee B, Panda SK, Sahoo L (2014) Cloning and functional characterization of a vacuolar Na+/H+ antiporter gene from mungbean (VrNHX1) and its ectopic expression enhanced salt tolerance in Arabidopsis thaliana. PLoS ONE 9:e106678
Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD (2016) Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev 82:371–406
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nagata T, Iizumi S, Satoh K, Kikuchi S (2008) Comparative molecular biological analysis of membrane transport genes in organisms. Plant Mol Biol 66:565–585
Nakamura A, Fukuda A, Sakai S, Tanaka Y (2006) Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol 47:32–42
Neuhaus HE, Trentmann O (2014) Regulation of transport processes across the tonoplast. Front Plant Sci 5:460
Nguyen VNT, Moon S, Jung KH (2014) Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J Plant Physiol 171:1276–1288
Nguyen CT, Agorio A, Jossier M, Depre S, Thomine S, Filleur S (2016) Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol 57:764–775
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosche M, Kangasjarvi J, Jiang X, Polle A (2005a) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139:1762–1772
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M, Kangasjärvi J, Jiang X, Polle A (2005b) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 1339:1762–1772
Pandolfi C, Mancuso S, Shabala S (2012) Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ Exp Bot 84:44–51
Pandolfi C, Azzarello E, Mancuso S, Shabala S (2016) Acclimation improves salt stress tolerance in Zea mays plants. J Plant Physiol 201:1–8
Pardo JM, Rubio F (2011) Na+ and K+ transporters in plant signaling. In: Geisler M, Venema K (eds) Transporters and pumps in plant signaling, signaling and communication in plants 7. Springer-Verlag, Berlin, pp 65–98
Parks GE, Dietrich MA, Schumaker KS (2002) Increased vacuolar Na+/H+ exchange activity in Salicornia bigelovii Torr. in response to salt. J Exp Bot 53:1055–1065
Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, Chen J, Qiu X, Zhu L, Zhang X, Auld D, Blumwald E, Zhang H, Gaxiola R, Payton P (2011) Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought and salt tolerance and increases fibre yield in the field conditions. Plant Biotech J 9:88–99
Pawlowicz I, Rapacz M, Perlikowski D, Gondek K, Kosmala A (2017) Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species. J Appl Genet 58:421–435
Pehlivan N, Sun L, Jarrett P, Yang X, Mishra N, Chen L, Kadioglu SG, Zhang H (2016) Co-overexpressing a plasma membrane and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic Arabidopsis plants. Plant Cell Physiol 57:1069–1084
Peng Y, Lin W, Cai W, Arora R (2007) Overexpression of a panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 226:729–740
Peng Z, He S, Sun J, Pan Z, Gong W, Lu Y, Du X (2016) Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci Rep 6:34548
Pitann B, Mohamed A, Neubert AB, Schubert S (2013) Tonoplast Na+/H+ antiporters of newly developed maize (Zea mays L.) hybrids contribute to salt resistance during the second phase of salt stress. J Plant Nutr Soil Sci 176:148–156
Pons R, Cornejo M, Sanz A (2011) Differential salinity-induced variations in the activity of H+-pumps and Na+/H+ antiporters that are involved in cytoplasm ion homeostasis as a function of genotype and tolerance level in rice cell lines. Plant Physiol Biochem 49:1399–1409
Porcel R, Aroca R, Azcon R, Ruiz-Lozano JM (2016) Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 26:673–684
Pottosin I, Dobrovinskaya O (2014) Non-selective cation channels in plasma and vacuolar membranes and their contribution to K+ transport. J Plant Physiol 171:732–742
Punia H, Tokas J, Bhadu S, Mohanty AK, Rawat P, Malik A, Satpal (2020) Proteome dynamics and transcriptome profiling in sorghum [Sorghum bicolor (L.) Moench] under salt stress. 3 Biotech 10:412
Punshon T, Hirschi K, Yang J, Lanzirotti A, Lai B, Guerinot ML (2012) The Role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed. Plant Physiol 158:352–362
Qiao G, Zhuo R, Liu M, Jiang J, Li H, Qiu W, Pan L, Lin S, Zhang X, Sun Z (2011) Over-expression of the Arabidopsis Na+/H+ antiporter gene in Populus deltoides CL X P. euramericana CL ‘“NL895”’ enhances its salt tolerance. Acta Physiol Plant 33:691–696
Qin H, Gu Q, Kuppu S, Sun L, Zhu X, Mishra N, Hu R, Shen G, Zhang J, Zhang Y, Zhu L, Zhang X, Burow M, Payton P, Zhang H (2013) Expression of the Arabidopsis vacuolar H+-pyrophosphatase gene AVP1 in peanut to improve drought and salt tolerance. Plant Biotech Rep 7:345–355
Qin Y, Wu W, Wang Y (2019) ZmHAK5 and ZmHAK1 function in K+ uptake and distribution in maize under low K+ conditions. J Intergr Plant Biol 61:691–705
Qiu QS, Guo Y, Quintero FJ, Pardo JM, Schumaker KS, Zhu JK (2004) Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J Biol Chem 279:207–215
Qiu N, Chen M, Guo J, Bao H, Ma X, Wang B (2007) Coordinate up-regulation of V-H+-ATPase and vacuolar Na+/H+ antiporter as a response to NaCl treatment in a C3 halophyte Suaeda salsa. Plant Sci 172:1218–1225
Qudeimat E, Faltusz AMC, Wheeler G, Lang D, Brownlee C, Reski R, Frank W (2008) A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc Natl Acad Sci (USA) 105:19555–19560
Queiros F, Fontes N, Silva P, Almeida D, Maeshima M, Geros H, Fidalgo F (2009) Activity of tonoplast proton pumps and Na+/H+ exchange in potato cell cultures is modulated by salt. J Exp Bot 60:1363–1374
Quiroga G, Erice G, Aroca R, Delgado-Huertas A, Ruiz-Lozano JM (2020) Elucidating the possible involvement of maize aquaporins and arbuscular mycorrhizal symbiosis in the plant ammonium and urea transport under drought stress conditions. Plants 9:148
Rahneshan R, Nasibi F, Lakehal A, Bellini C (2018) Unravelling salt stress responses in two pistachio (Pistacia vera L.) genotypes. Acta Physiol Plant 40:172
Rhee J, Horie T, Sasano S, Nakahara Y, Katsuhara M (2017) Identification of an H2O2 permeable PIP aquaporin in barley and a serine residue promoting H2O2 transport. Physiol Plant 159:120–128
Rodriguez-Rosales MP, Jiang X, Gálvez FJ, Aranda MN, Cubero B, Venema K (2008) Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol 179:366–377
Rubio F, Nieves-Cordones M, Horie T, Shabala S (2020) Doing ‘business as usual’ comes with a cost: evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol 225:1097–1104
Rudnytska MV, Palladina TA (2017) Effect of preparations Methyure and Ivine on Ca2+-ATPases activity in plasma and vacuolar membrane of corn seedling roots under salt stress conditions. Ukr Biochem J 89:76–81
SadeN VBJ, Diber A, Shatil A, Ronen G (2009) Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181:651–661
Saha J, Sengupta A, Gupta K, Gupta B (2015) Molecular phylogenetic study and expression analysis of ATP-binding cassette transporter gene family in Oryza sativa in response to salt stress. Comput Biol Chem 54:18–32
Saibi W, Brini F (2021) Ion transporters and their molecular regulation mechanism in plants. J Plant Sci Phytopath 5:28–43
Sambe MAN, He XY, Tu QH, Guo ZF (2015) A cold-induced myo-inositol transporter-like gene confers tolerance to multiple abiotic stresses in transgenic tobacco plants. Physiol Plant 53:355–364
Santander C, Aroca R, Cartes P, Vidal G, Cornejo P (2020) Aquaporins and cation transporters are differentially regulated by two arbuscular mycorrhizal fungi strains in lettuce cultivars growing under salinity conditions. Plant Physiol Biochem 158:396–409
Sardans J, Peñuelas J (2021) Potassium control of plant functions: ecological and agricultural implications. Plants 10:419
Sathee L, Sairam K, Chinnusamy V, Jha SK (2015) Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiol Plant 37:169
Schilling RK, Marschner P, Shavrukov Y, Berger B, Tester M, Roy SJ, Plett DC (2014) Expression of the Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) improves the shoot biomass of transgenic barley and increases grain yield in a saline field. Plant Biotech J 12:378–386
Schneider S, Beyhl D, Hedrich R, Sauera N (2008) Functional and physiological characterization of Arabidopsis INOSITOL TRANSPORTER1, a novel tonoplast-localized transporter for myo-inositol. Plant Cell 20:1073–1087
Schönknecht G (2013) Calcium signals from the vacuole. Plants 2:589–614
Seifikalhor M, Aliniaeifard S, Shomali A, Azad N, Hassan B, Lastochkin O, Li T (2019) Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal Behav 14:11
Senadheera P, Singh RK, Maathuis FJM (2009) Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J Exp Bot 60:2553–2563
Shabala S, Cuin TA, Pang JY, Pang J, Chen Z, Conn S, Wegner LH (2010) Xylem ionic relations and salinity tolerance in barley. Plant J 61:839–853
Shabala L, Zhang J, Pottosin I, Bose J, Zhu M, Fuglsang AT, Velarde-Buendia A, Massart A, Hill CB, Roessner U, Bacic A, Wu H, Azzarello E, Pandolfi C, Zhou M, Poschenrieder C, Mancuso S, Shabala S (2016) Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol 172:2445–2458
Shabala S, Chen G, Chen Z, Pottosin I (2020) The energy cost of the tonoplast futile sodium leak. New Phytol 225:1105–1110
Shen G, Wei J, Qiu X, Hu R, Kuppu S, Alud D, Blumwald E, Gaxiola R, Pyton P, Zhang H (2015) Co-overexpression of AVP1 and AtNHX1 in cotton further improves drought and salt tolerance in transgenic cotton plants. Plant Mol Biol Rep 33:167–177
Shi M, Wang S, Zhang Y, Wang S, Zhao J, Feng H, Sun P, Fang C, Xie X (2020) Genome-wide characterization and expression analysis of ATP-binding cassette (ABC) transporters in strawberry reveal the role of FvABCC11 in cadmium tolerance. Sci Hortic 271:109464
Shi H, Zhu J (2002) Regulation of expression of the vacuolar Na+/H+ antiporter gene AtNHX1 by salt stress and ABA. Plant Mol Biol 50:543–550
Silva P, Gerós H (2009) Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signal Behav 4:718–726
Silva P, Facanha AR, Tavares RM, Geros H (2010) Role of tonoplast proton pumps and Na+/H+ antiport system in salt tolerance of Populus euphratica oliv. J Plant Growth Regul 29:23–34
Singh RK, Deshmukh R, Muthamilarasan M, Rani R, Prasad M (2020) Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol Biochem 149:178–189
Sobreira ACM, Maia Y, Reboušas DM, Fontenele NMB, Costa JH, Otoch MLO, Oliveira LMN, Orellano EG, Melo DF (2014) Vacuolar proton pumps regulation during development of Vigna unguiculata seedlings under salt stress. Theor Exp Plant Physiol 26:167–175
Solis CA, Yong M, Venkataraman G, Milham P, Zhou M, Shabala L, Holford P, Shabala S, Chen Z (2021) Sodium sequestration confers salinity tolerance in an ancestral wild rice. Physiol Plant 17:1594–1608
Su J, Bai T, Wang F, Bao A (2019) Overexpression of Arabidopsis H+-pyrophosphatase improves the growth of alfalfa under long-term salinity, drought conditions and phosphate deficiency. Czech J Genetics Plant Breed 55:156–161
Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009) NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149:1141–1153
Sun T, Fan L, Yang J, Cao R, Yang C, Zhang J, Wang D (2019) A glycine max sodium/hydrogen exchanger enhances salt tolerance through maintaining higher Na+ efflux rate and K+/Na+ ratio in Arabidopsis. BMC Plant Biol 19:469
Tang R, Li C, Xu K, Du Y, Xia T (2010) Isolation, functional characterization and expression pattern of vacuolar Na+/H+ antiporter gene TrNHX1 from Trifolium repens L. Plant Mol Biol Rep 28:102–111
Tang R, Yang Y, Yang L, Liu H, Wang C, Yu M, Gao X, Zhang H (2014) Poplar calcineurin B-like proteins PtCBL10A and PtCBL10B regulate shoot salt tolerance through interaction with PtSOS2 in the vacuolar membrane. Plant Cell Environ 37:573–588
Tealle NL, Tyerman SD (2010) Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Theerawitaya C, Tisarum R, Samphumphuang T, Takabe T, Cha-um S (2020) Expression levels of the Na+/K+ transporter OsHKT2;1 and vacuolar Na+/H+ exchanger OsNHX1, Na enrichment, maintaining the photosynthetic abilities and growth performances of indica rice seedlings under salt stress. Physiol Mol Biol Plants 26:513–523
Theerawitaya C, Samphumphuang T, Tisarum R, Siangliw M, Cha-um S, Takabe T, Toojinda T (2021) Transcriptional expression of Na+ homeostasis-related genes and physiological responses of rice seedlings under salt stress. J Plant Biochem Biotech 30:81–91
Tsiantis MS, Bartholomew DM, Smith JA (1996) Salt regulation of transcript levels for the c subunit of a leaf vacuolar H(+)-ATPase in the halophyte Mesembryanthemum crystallinum. Plant J 9:729–736
Upadhyay A, Upadhyay AK, Bhirangi RA (2012) Expression of Na+/H+ antiporter gene in response to water and salinity stress in grapevine rootstocks. Biol Plant 56:762–766
Voelker C, Gomez-Porras JL, Becker D, Hamamoto S, Uozumi N, Gambale F, Mueller-Roeber B, Czempinski K, Dreyer I (2010) Roles of tandem-pore K+ channels in plants—a puzzle still to be solved. Plant Biol 12:56–63
Wang B, Lüttge U, Ratajczak R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot 52:2355–2365
Wang Y, Xu H, Zhang G, Zhu H, Zhang L, Zhang Z (2009) Expression and responses to dehydration and salinity stresses of V-PPase gene members in wheat. J Genet Genomics 36:711–720
Wang L, He X, Zhao Y, Shen Y, Huang Z (2011a) Wheat vacuolar H+-ATPase subunit B cloning and its involvement in salt tolerance. Planta 234:1–7
Wang X, Li Y, Ji W (2011b) A novel Glycine soja tonoplast intrinsic protein gene responds to abiotic stress and depresses salt and dehydration tolerance in transgenic Arabidopsis thaliana. J Plant Physiol 168:1241–1248
Wang S, Su SZ, Wu Y, Li SP, ShanLiu XHHK, Wang S, Yuan YP (2015) Overexpression of maize chloride channel gene ZmCLC-d in Arabidopsis thaliana improved its stress resistance. Biol Plant 59:55–64
Wang L, Ma YK, Li NN, Zhang WB, Mao HP, Lin XF (2016a) Isolation and characterization of a tonoplast Na+/H+ antiporter from the halophyte Nitraria sibirica. Biol Plant 60:113–122
Wang C, Xu WT, Jin HL, Zhang TJ, Lai JB, Zhou X, Zhang SC, Liu SJ, Duan XW, Wang HB (2016b) A putative chloroplast-localized Ca2+/H+ antiporter CCHA1 is involved in calcium and pH homeostasis and required for PSII function in Arabidopsis. Mol Plant 9:1183–1196
Wang G, Bi A, Amombo E, Li H, Zhang L, Cheng C, Hu T, Fu J (2017b) Exogenous calcium enhances the photosystem II photochemistry response in salt stressed tall fescue. Front Plant Sci 8:2032
Wang P, Guo Y, Wang Y, Gao C (2020) Vacuolar membrane H+-ATPase cˋˋ subunit gene (ThVHAcˋˋ1) from Tamarix hispida willd improves salt stress tolerance. Plant Physiol Biochem 157:370–378
Wang N, Qi H, Qiao W, Shi J, Xu Q, Zhou H, Yan G, Huang Q (2017) Cotton (Gossypium hirsutum L.) genotypes with contrasting K+/Na+ ion homeostasis: implications for salinity tolerance. Acta Physiol Plant 39:77
Wanke D, Kolukisaoglu HU (2010) An update on the ABCC transporter family in plants: many genes, many proteins, but how many functions? Plant Biol 12:15–25
Wege S, Gilliham M, Henderson SW (2017) Chloride: not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J Exp Bot 68:3057–3069
Wei P, Wang L, Liu A, Yu B, Lam H (2016) GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front Plant Sci 7:1082
Wei P, Che P, Shen L, Cui Y, Wu S, Cheng C, Liu F, Li M, Yu B, Lam H (2019) Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol 19:121
White PJ, Smith JAC (1989) Proton and anion transport at the tonoplast in crassulacean-acid-metabolism plants: specificity of the malate-influx system in Kalanchoe daigremontiana. Planta 179:265–274
Wong TH, Li MW, Yao XQ, Lam HM (2013) The GmCLC1 protein from soybean functions as a chloride ion transporter. J Plant Physiol 170:101–104
Wu H, Li Z (2019) The importance of Cl− exclusion and vacuolar Cl− sequestration: revisiting the role of Cl− transport in plant salt tolerance. Front Plant Sci 10:1418
Wu H, Shabala L, Liu X, Azzarello E, Zhou M, Pandolfi C, Chen Z, Bose J, Mancuso S, Shabala S (2015) Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Front Plant Sci 6:71
Wu H, Zhang X, Giraldo JP, Shabala S (2018) It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 431:1–17
Wu H, Shabala L, Zhou M, Su N, Wu Q, Ul-Haq T, Zhu J, Mancuso S, Azzarello E, Shabala S (2019) Root vacuolar Na+ sequestration but not exclusion from uptake correlates with barley salt tolerance. Plant J 100:55–67
Wu XX, Li J, Wu XD, Liu Q, Wang ZK, Liu SS, Li SN, Ma YL, Sun J, Zhao L, Li HY, Li DM, Li WB, Su AY (2016) Ectopic expression of Arabidopsis thaliana Na+(K+)/H+ antiporter gene, AtNHX5, enhances soybean salt tolerance. Genet Mol Res 15:gmr7483
Xin S, Yu G, Sun L, Qiang X, Xu N, Cheng X (2014) Expression of tomato SlTIP2;2 enhances the tolerance to salt stress in the transgenic Arabidopsis and interacts with target proteins. J Plant Res 127:695–708
Xu CX, Zheng L, Gao CQ, Wang C, Liu GF, Jiang J, Wang YC (2011) Overexpression of a vacuolar H+-ATPase c subunit gene mediates physiological changes leading to enhanced salt tolerance in transgenic tobacco. Plant Mol Biol Rep 29:424–430
Xu C, Wang M, Zhou L, Quan T, Xia G (2013) Heterologous expression of the wheat aquaporin gene TaTIP2;2 compromises the abiotic stress tolerance of Arabidopsis thaliana. PLoS One 8:9618
Xue Z, Zhi D, Xue G, Zhang H, Zhao Y, Xi G (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Yaish M (2015) Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet Mol Res 14:9943–9950
Yan Y, Sun M, Li Y, Wang J, He C, Yu X (2020) The CsGPA1-CsAQPs module is essential for salt tolerance of cucumber seedlings. Plant Cell Rep 39:1301–1316
Yang L, Liu H, Fu SM, Ge HM, Tang RJ, Yang Y, Wang HH, Zhang HX (2017) Na+/H+ and K+/H+ antiporters AtNHX1 and AtNHX3 from Arabidopsis improve salt and drought tolerance in transgenic poplar. Biol Plant 61:641–650
YangY TRJ, Li B, Wang HH, Jin YL, Jiang CM, Bao Y, Su HY, Zhao N, Ma XJ (2015) Overexpression of a Populus trichocarpa H+-pyrophsophatase gene PtVP1.1 confers salt tolerance on transgenic poplar. Tree Physiol 35:663–677
Yarra R, Kirti PB (2019) Expressing class I wheat NHX (TaNHX2) gene in eggplant (Solanum melongena L.) improves plant performance under saline condition. Funct Integr Genomics 19:541–554
Yeo AR (2006) Salinity resistance: physiologies and prices. Physiol Plant 58:214–222
Yousefirad S, Soltanloo H, Ramezanpour SS, Zaynalinezhad K, Shariati V (2018) Salt oversensitivity derived from mutation breeding improves salinity tolerance in barley via ion homeostasis. Biol Plant 62:775–785
Yu J, Huang J, Wang Z, Zhang J, Chen SY (2007) An Na+/H+ antiport gene from wheat plays an important role in stress tolerance. J Biosci 32:1153–1161
Yuan H, Ma Q, Wu G, Wang P, Hu J, Wang S (2015) ZxNHX controls Na+ and K+ homeostasis at the whole-plant level in Zygophyllum xanthoxylum through feedback regulation of the expression of genes involved in their transport. Ann Bot 115:495–507
Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:24.1-24.31
Zeng Y, Li Q, Wang H, Zhang J, Du J, Feng H, Blumwald E, Yu L, Xu G (2018) Two NHX-type transporters from Helianthus tuberosus improve the tolerance of rice to salinity and nutrient deficiency stress. Plant Biotech J 16:310–321
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang XK, Zhou QH, Cao JH, Yu BJ (2011) Differential Cl-/salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their hybrid seedlings. J Agron Crop Sci 197:329–339
Zhang YM, Zhang HM, Liu ZH, Li HC, Guo XL, Li GL (2015a) The wheat NHX antiporter gene TaNHX2 confers salt tolerance in transgenic alfalfa by increasing the retention capacity of intracellular potassium. Plant Mol Biol 87:317–327
Zhang C, Hicks GR, Raikhel NV (2015b) Molecular composition of plant vacuoles: important but less understood regulations and roles of tonoplast lipids. Plants 4:320–333
Zhang W, Bao PWZ, Ma Q, Duan L, Bao A, Zhang J, Wang S (2017a) SOS1, HKT1;5, and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Front Plant Sci 8:576
Zhang W, Meng J, Xing J, Yang S, Guo F, Li X, Wan S (2017b) The K+/H+ antiporter AhNHX1 improved tobacco tolerance to NaCl stress by enhancing K+ retention. J Plant Biol 60:259–267
Zhang M, Chen Q, Zhou P, Zhang Q, Fang Y (2018) NaCl-induced changes in vacuolar H+-ATPase expression and vacuolar membrane lipid composition of two shrub willow clones differing in their response to salinity. Plant Growth Reg 286:445–453
Zhang Z, Tong T, Fang Y, Zheng J, Zhang X, Niu C, Li J, Zhang X, Xue D (2020a) Genome-wide identification of barley ABC genes and their expression in response to abiotic stress treatment Zhang. Plants 9:1281
Zhang X, Zorb C, Geilfus C (2020b) The root as a sink for chloride under chloride-salinity. Plant Physiol Biochem 155:161–168
Zhang J, Xiao Q, Guo T, Wang P (2021) Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil. Open Chem 19:9–22
Zhang M, Fang Y, Liang Z, Huang L (2012) Enhanced expression of vacuolar H+-ATPase subunit E in the roots is associated with the adaptation of Broussonetia papyrifera to salt stress. PLoS One 7:8183
Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H (2006) Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol Breed 17:341–353
Zhao Q, Zhao YJ, Zhao BC (2009) Cloning and functional analysis of wheat V-H+-ATPase subunit genes. Plant Mol Biol 69:33–46
Acknowledgements
I am grateful to Ms. Nada Mansour for preparing the manuscript figures
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MMFM proposed the idea of the review, performed the literature search and data analysis, drafted the manuscript, and critically revised the review. I certify that this paper has not been submitted elsewhere.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Handling Editor: Antonella Locascio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansour, M.M.F. Role of Vacuolar Membrane Transport Systems in Plant Salinity Tolerance. J Plant Growth Regul 42, 1364–1401 (2023). https://doi.org/10.1007/s00344-022-10655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10655-9